Advancing aquaculture: Production of xenogenic catfish by transplanting blue catfish (Ictalurus furcatus) and channel catfish (I. punctatus) stem cells into white catfish (Ameiurus catus) triploid fry
- PMID: 38848398
- PMCID: PMC11161074
- DOI: 10.1371/journal.pone.0302687
Advancing aquaculture: Production of xenogenic catfish by transplanting blue catfish (Ictalurus furcatus) and channel catfish (I. punctatus) stem cells into white catfish (Ameiurus catus) triploid fry
Abstract
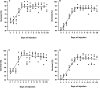
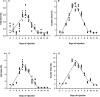
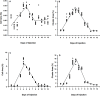
Xenogenesis has been recognized as a prospective method for producing channel catfish, Ictalurus punctatus ♀ × blue catfish, I. furcatus ♂ hybrids. The xenogenesis procedure can be achieved by transplanting undifferentiated stem cells derived from a donor fish into a sterile recipient. Xenogenesis for hybrid catfish embryo production has been accomplished using triploid channel catfish as a surrogate. However, having a surrogate species with a shorter maturation period, like white catfish (Ameiurus catus), would result in reduced feed costs, labor costs, and smaller body size requirements, making it a more suitable species for commercial applications where space is limited, and as a model species. Hence, the present study was conducted to assess the effectiveness of triploid white catfish as a surrogate species to transplant blue catfish stem cells (BSCs) and channel catfish stem cells (CSCs). Triploid white catfish fry were injected with either BSCs or CSCs labeled with PKH 26 fluorescence dye from 0 to 12 days post hatch (DPH). No significant differences in weight and length of fry were detected among BSCs and CSCs injection times (0 to 12 DPH) when fry were sampled at 45 and 90 DPH (P > 0.05). The highest survival was reported when fry were injected between 4.0 to 5.5 DPH (≥ 81.2%). At 45 and 90 DPH, cell and cluster area increased for recipients injected from 0 to 5.2 DPH, and the highest cluster area values were reported between 4.0 to 5.2 DPH. Thereafter, fluorescent cell and cluster area in the host declined with no further decrease after 10 DPH. At 45 DPH, the highest percentage of xenogens were detected when fry were injected with BSCs between 4.0 to 5.0 and CSCs between 3.0 to 5.0 DPH. At 90 DPH, the highest number of xenogens were detected from 4.0 to 6.0 DPH when injected with either BSCs or CSCs. The current study demonstrated the suitability of white catfish as a surrogate species when BSCs and CSCs were transplanted into triploid white catfish between 4.0 to 6.0 DPH (27.4 ± 0.4°C). Overall, these findings allow enhanced efficiency of commercializing xenogenic catfish carrying gametes of either blue catfish or channel catfish.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Development of a spermatogonia cryopreservation protocol for blue catfish, Ictalurus furcatus.Cryobiology. 2020 Dec;97:46-52. doi: 10.1016/j.cryobiol.2020.10.010. Epub 2020 Oct 13. Cryobiology. 2020. PMID: 33058900
-
Testicular germ line cell identification, isolation, and transplantation in two North American catfish species.Fish Physiol Biochem. 2018 Apr;44(2):717-733. doi: 10.1007/s10695-018-0467-3. Epub 2018 Jan 22. Fish Physiol Biochem. 2018. PMID: 29357082
-
High-throughput cryopreservation of spermatozoa of blue catfish (Ictalurus furcatus): Establishment of an approach for commercial-scale processing.Cryobiology. 2011 Feb;62(1):74-82. doi: 10.1016/j.cryobiol.2010.12.006. Epub 2010 Dec 19. Cryobiology. 2011. PMID: 21176772 Free PMC article.
-
Response of cecropin transgenesis to challenge with Edwardsiella ictaluri in channel catfish Ictalurus punctatus.Fish Shellfish Immunol. 2022 Jul;126:311-317. doi: 10.1016/j.fsi.2022.05.050. Epub 2022 May 27. Fish Shellfish Immunol. 2022. PMID: 35636698 Review.
-
Catfish Biology and Farming.Annu Rev Anim Biosci. 2018 Feb 15;6:305-325. doi: 10.1146/annurev-animal-030117-014646. Epub 2017 Nov 6. Annu Rev Anim Biosci. 2018. PMID: 29106819 Review.
References
-
- NOAA (National Oceanic and Atmospheric Administration). 2020. Global aquaculture URL Fisheries of the United States, 2020 | NOAA Fisheries (accessed February 2023).
-
- Dunham RA, Brummett RE. Response of two generations of selection to increased body weight in channel catfish, Ictalurus punctatus, compared to hybridization with blue catfish, I. furcatus, males. Journal of Applied Aquaculture. 1999. Sep 1;9(3):37–45.
-
- Brown TW, Chappell JA, Boyd CE. A commercial-scale, in-pond raceway system for Ictalurid catfish production. Aquacultural engineering. 2011. May 1;44(3):72–9.
-
- Arias CR, Cai W, Peatman E, Bullard SA. Catfish hybrid Ictalurus punctatus × I. furcatus exhibits higher resistance to columnaris disease than the parental species. Diseases of Aquatic Organisms. 2012. Aug 13;100(1):77–81. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

