Race and Remdesivir: Examination of Clinical Outcomes in a Racially and Ethnically Diverse Cohort in New York City
- PMID: 38846265
- PMCID: PMC11152153
- DOI: 10.18865/1653
Race and Remdesivir: Examination of Clinical Outcomes in a Racially and Ethnically Diverse Cohort in New York City
Abstract
Objective: To compare clinical characteristics and examine in-hospital length of stay (LOS) differences for COVID-19 patients who received remdesivir, by race or ethnicity.
Design: Retrospective descriptive analysis comparing cumulative LOS as a proxy of recovery time.
Setting: A large academic medical center serving a minoritized community in Northern Manhattan, New York City.
Participants: Inpatients (N=1024) who received remdesivir from March 30, 2020-April 20, 2021.
Methods: We conducted descriptive analyses among patients who received remdesivir. Patients were described by proxies of social determinants of health (SDOH): race and ethnicity, residence, insurance coverage, and clinical characteristics. We calculated median hospital LOS as the cumulative incidence of hospitalized patients who were discharged alive, and tested differences between groups by using the Gray test. Patients who died or were discharged to hospice were censored at 29 days.
Main outcome measures: The primary outcome was hospital LOS. The secondary outcome was in-hospital mortality.
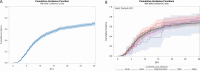
Results: Median LOS was 11.9 days (95% CI, 10.8-13.2) overall, with Black patients having the shortest (10.0 days, 95% CI, 8.0-13.2) and Asian patients having the longest (16.2 days, 95% CI, 8.3-27.2) LOS. A total of 214 patients (21%) died or were discharged to hospice, ranging from 16.5% to 23.7% of patients who identified as Black and Other (multiracial, biracial, declined), respectively.
Conclusions: COVID-19 has disproportionately burdened communities of color. We observed no difference in median LOS between racial or ethnic groups, which supports the notion that the heterogeneous effect of remdesivir in the literature may be explained in part by underrecruitment or participation of Black, Hispanic, and Asian patients in clinical trials.
Keywords: COVID-19; Ethnicity; Length of Stay; Race; Remdesivir.
Conflict of interest statement
Conflict of Interest No conflicts of interest reported by authors.
Figures
Similar articles
-
Comparison of Time to Clinical Improvement With vs Without Remdesivir Treatment in Hospitalized Patients With COVID-19.JAMA Netw Open. 2021 Mar 1;4(3):e213071. doi: 10.1001/jamanetworkopen.2021.3071. JAMA Netw Open. 2021. PMID: 33760094 Free PMC article.
-
Association of Remdesivir Treatment With Survival and Length of Hospital Stay Among US Veterans Hospitalized With COVID-19.JAMA Netw Open. 2021 Jul 1;4(7):e2114741. doi: 10.1001/jamanetworkopen.2021.14741. JAMA Netw Open. 2021. PMID: 34264329 Free PMC article.
-
Remdesivir for the Treatment of Severe COVID-19: A Community Hospital's Experience.J Am Osteopath Assoc. 2020 Dec 1;120(12):926-933. doi: 10.7556/jaoa.2020.156. J Am Osteopath Assoc. 2020. PMID: 33136164
-
Remdesivir in Coronavirus Disease 2019 (COVID-19) treatment: a review of evidence.Infection. 2021 Jun;49(3):401-410. doi: 10.1007/s15010-020-01557-7. Epub 2021 Jan 2. Infection. 2021. PMID: 33389708 Free PMC article. Review.
-
Remdesivir in COVID-19: A critical review of pharmacology, pre-clinical and clinical studies.Diabetes Metab Syndr. 2020 Jul-Aug;14(4):641-648. doi: 10.1016/j.dsx.2020.05.018. Epub 2020 May 12. Diabetes Metab Syndr. 2020. PMID: 32428865 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous