P-hydroxybenzaldehyde protects Caenorhabditis elegans from oxidative stress and β-amyloid toxicity
- PMID: 38841104
- PMCID: PMC11150654
- DOI: 10.3389/fnagi.2024.1414956
P-hydroxybenzaldehyde protects Caenorhabditis elegans from oxidative stress and β-amyloid toxicity
Abstract
Introduction: Gastrodia elata is the dried tuber of the orchid Gastrodia elata Bl. It is considered a food consisting of a source of precious medicinal herbs, whose chemical composition is relatively rich. Gastrodia elata and its extracted fractions have been shown to have neuroprotective effects. P-hydroxybenzaldehyde (p-HBA), as one of the main active components of Gastrodia elata, has anti-inflammatory, antioxidative stress, and cerebral protective effects, which has potential for the treatment of Alzheimer's disease (AD). The aim of this study was to verify the role of p-HBA in AD treatment and to investigate its mechanism of action in depth based using the Caenorhabditis elegans (C. elegans) model.
Methods: In this study, we used paralysis, lifespan, behavioral and antistress experiments to investigate the effects of p-HBA on AD and aging. Furthermore, we performed reactive oxygen species (ROS) assay, thioflavin S staining, RNA-seq analysis, qPCR validation, PCR Array, and GFP reporter gene worm experiment to determine the anti-AD effects of p-HBA, as well as in-depth studies on its mechanisms.
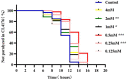
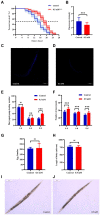
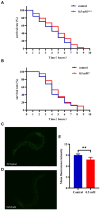
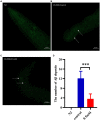
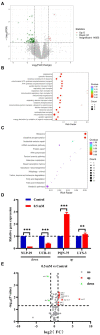
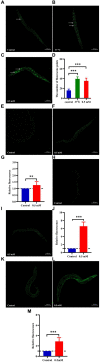
Results: p-HBA was able to delay paralysis, improve mobility and resistance to stress, and delay aging in the AD nematode model. Further mechanistic studies showed that ROS and lipofuscin levels, Aβ aggregation, and toxicity were reduced after p-HBA treatment, suggesting that p-HBA ameliorated Aβ-induced toxicity by enhancing antioxidant and anti-aging activity and inhibiting Aβ aggregation. p-HBA had a therapeutic effect on AD by improving stress resistance, as indicated by the down-regulation of NLP-29 and UCR-11 expression and up-regulation of PQN-75 and LYS-3 expression. In addition, the gene microarray showed that p-HBA treatment played a positive role in genes related to AD, anti-aging, ribosomal protein pathway, and glucose metabolism, which were collectively involved in the anti-AD mechanism of p-HBA. Finally, we also found that p-HBA promoted nuclear localization of DAF-16 and increased the expression of SKN-1, SOD-3, and GST-4, which contributed significantly to inhibition of Aβ toxicity and enhancement of antioxidative stress.
Conclusion: Our work suggests that p-HBA has some antioxidant and anti-aging activities. It may be a viable candidate for the treatment and prevention of Alzheimer's disease.
Keywords: Alzheimer’s disease; Aβ protein; Caenorhabditis elegans; neuroprotection; oxidative stress; p-hydroxybenzaldehyde.
Copyright © 2024 Yu, Tao, Xiao and Duan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
4,4'-methylenediphenol reduces Aβ-induced toxicity in a Caenorhabditis elegans model of Alzheimer's disease.Front Aging Neurosci. 2024 May 30;16:1393721. doi: 10.3389/fnagi.2024.1393721. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38872629 Free PMC article.
-
Ethyl acetate extract of Gastrodia elata protects Caenorhabditis elegans from oxidative stress and amyloid β peptide toxicity.Exp Ther Med. 2023 Jul 7;26(2):405. doi: 10.3892/etm.2023.12104. eCollection 2023 Aug. Exp Ther Med. 2023. PMID: 37522064 Free PMC article.
-
Para-Hydroxybenzyl Alcohol Delays the Progression of Neurodegenerative Diseases in Models of Caenorhabditis elegans through Activating Multiple Cellular Protective Pathways.Oxid Med Cell Longev. 2022 Mar 31;2022:8986287. doi: 10.1155/2022/8986287. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35401930 Free PMC article.
-
Transgenic C. elegans as a model in Alzheimer's research.Curr Alzheimer Res. 2005 Jan;2(1):37-45. doi: 10.2174/1567205052772768. Curr Alzheimer Res. 2005. PMID: 15977988 Review.
-
Therapeutic potentials of plant iridoids in Alzheimer's and Parkinson's diseases: A review.Eur J Med Chem. 2019 May 1;169:185-199. doi: 10.1016/j.ejmech.2019.03.009. Epub 2019 Mar 8. Eur J Med Chem. 2019. PMID: 30877973 Review.
Cited by
-
Herbal medicines in Alzheimer's disease and the involvement of gut microbiota.Front Pharmacol. 2024 Jul 16;15:1416502. doi: 10.3389/fphar.2024.1416502. eCollection 2024. Front Pharmacol. 2024. PMID: 39081953 Free PMC article. Review.
-
Clonostachys rosea, a Pathogen of Brown Rot in Gastrodia elata in China.Biology (Basel). 2024 Sep 17;13(9):730. doi: 10.3390/biology13090730. Biology (Basel). 2024. PMID: 39336157 Free PMC article.
References
-
- Alafuzoff I., Pikkarainen M., Arzberger T., Thal D. R., Al-Sarraj S., Bell J., et al. . (2008). Inter-laboratory comparison of neuropathological assessments of beta-amyloid protein: a study of the BrainNet Europe consortium. Acta Neuropathol. 115, 533–546. doi: 10.1007/s00401-008-0358-2, PMID: - DOI - PubMed
-
- Bin X., Chun X., Ting S., Shi J., Qing L., Xiu-fang L. (2017). 4-hydroxybenzyl aldehyde can prevent the acute cerebral ischemic injury in rats. Chin. Tradit. Patent Med. 39, 1572–1576. doi: 10.3969/j.issn.1001-1528.2017.08.005 - DOI
-
- Bountagkidou O. G., Ordoudi S. A., Tsimidou M. Z. (2010). Structure–antioxidant activity relationship study of natural hydroxybenzaldehydes using in vitro assays. Food Res. Int. 43, 2014–2019. doi: 10.1016/j.foodres.2010.05.021 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

