Yttrium-90 anti-CD25 BEAM conditioning for autologous hematopoietic cell transplantation in Peripheral T-cell lymphoma
- PMID: 38838232
- PMCID: PMC11415869
- DOI: 10.1182/bloodadvances.2023012497
Yttrium-90 anti-CD25 BEAM conditioning for autologous hematopoietic cell transplantation in Peripheral T-cell lymphoma
Abstract
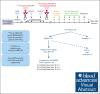
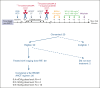
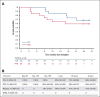
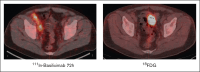
Peripheral T-cell lymphomas (PTCLs) have a poor prognosis with current treatments. High-dose chemotherapy followed by autologous hematopoietic cell transplant (AHCT) is used as a consolidation strategy after achieving clinical remission with first-line therapy, as well as in chemotherapy-sensitive relapse if allogeneic transplant is not an option. CD25 is a targetable protein often highly expressed in PTCLs. In this phase 1 clinical trial, we tested the addition of β-emitting 90yttrium (90Y)-labeled chimeric anti-CD25 basiliximab (aTac) to BEAM (carmustine, etoposide, cytarabine, and melphalan) as conditioning for AHCT for patients with PTCL. Twenty-three AHCT-eligible patients were enrolled, and 20 received therapeutic 90Y-aTac-BEAM AHCT. Radiation doses of 0.4, 0.5, and 0.6 mCi/kg were tested. With no observed dose-limiting toxicities, 0.6 mCi/kg was deemed the recommended phase 2 dose. The most prevalent adverse effect, grade 2 mucositis, was experienced by 80% of patients. As of this report, 6 (30%) of the treated patients had died, 5 due to progressive disease and 1 due to multiple organ failure (median time of death, 17 months [range, 9-21]) after AHCT. Median follow-up was 24 months (range, 9-26) overall and 24 months (range, 13-26) for surviving patients. For patients who received therapeutic 90Y-aTac-BEAM AHCT, the 2-year progression-free and overall survival were 59% (95% confidence interval [CI], 34-77) and 68% (95% CI, 42-84), respectively. 90Y-aTac-BEAM appears to be safe as an AHCT conditioning regimen for PTCL, with no increased toxicity over the toxicities historically seen with BEAM alone in this patient population. This trial was registered at www.ClinicalTrials.gov as #NCT02342782.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.Z. is a consultant for Kyowa Kirin, Seattle Genetics, Verastem, Daiichi Sankyo, and Mundi Pharma; serves of the speakers bureau of Seattle Genetics, SecureBio, Daiichi Sankyo, and AbbVie; and reports research support from Seattle Genetics, SecureBio, Daiichi Sankyo, and AbbVie. J.W. reports grant support from RefleXion Inc, Varian Inc, Accuray Inc, Telix Inc, and Blue Earth Diagnostics, Inc. A.F.H. reports research funding from Bristol Myers Squibb, Merck, Genentech, Inc, F. Hoffmann-La Roche Ltd, Gilead Sciences, Seattle Genetics, AstraZeneca, and ADC Therapeutics; and reports consultancy with Bristol Myers Squibb, Merck, Genentech, Inc, F. Hoffmann-La Roche Ltd, Kite Pharma/Gilead, Seattle Genetics, Karyopharm, Takeda, Tubulis, and AstraZeneca. S.D. reports research funding from Bayer. A.M.W. reports consultancy and board membership with ImaginAb; and reports consultancy with AstraZeneca, and Novartis Institute for Biomedical Research. The remaining authors declare no competing financial interests.
Figures
Similar articles
-
Prognostic differences between carmustine, etoposide, cytarabine and melphalan (BEAM) and carmustine, etoposide, cytarabine, melphalan and fludarabine (BEAMF) regimens before autologous stem cell transplantation plus chimeric antigen receptor T therapy in patients with refractory/relapsed B-cell non-Hodgkin-lymphoma.Cytotherapy. 2024 May;26(5):456-465. doi: 10.1016/j.jcyt.2024.01.012. Epub 2024 Feb 19. Cytotherapy. 2024. PMID: 38385909
-
Early Toxicity and Efficacy of Four Different Conditioning Regimens for Autologous Hematopoietic Cell Transplantation in Patients With Lymphoma: Impact of Drug Shortages in a Resource-Constrained Country.Transplant Cell Ther. 2024 Oct;30(10):1003.e1-1003.e9. doi: 10.1016/j.jtct.2024.07.025. Epub 2024 Aug 2. Transplant Cell Ther. 2024. PMID: 39097096
-
90Y-Daclizumab (Anti-CD25), High-Dose Carmustine, Etoposide, Cytarabine, and Melphalan Chemotherapy and Autologous Hematopoietic Stem Cell Transplant Yielded Sustained Complete Remissions in 4 Patients with Recurrent Hodgkin's Lymphoma.Cancer Biother Radiopharm. 2020 May;35(4):249-261. doi: 10.1089/cbr.2019.3298. Epub 2020 Apr 9. Cancer Biother Radiopharm. 2020. PMID: 32275165 Free PMC article. Clinical Trial.
-
Novel regimens prior to autologous stem cell transplantation for the management of adults with relapsed/refractory non-Hodgkin lymphoma and Hodgkin lymphoma: alternatives to BEAM conditioning.Leuk Lymphoma. 2016 Nov;57(11):2499-509. doi: 10.1080/10428194.2016.1185785. Epub 2016 May 31. Leuk Lymphoma. 2016. PMID: 27243412 Review.
-
Peripheral T-cell lymphoma: the role of hematopoietic stem cell transplantation.Crit Rev Oncol Hematol. 2014 Feb;89(2):248-61. doi: 10.1016/j.critrevonc.2013.08.016. Epub 2013 Sep 8. Crit Rev Oncol Hematol. 2014. PMID: 24075060 Review.
References
-
- Kharfan-Dabaja MA, Kumar A, Ayala E, et al. Clinical practice recommendations on indication and timing of hematopoietic cell transplantation in mature T cell and NK/T cell lymphomas: an international collaborative effort on behalf of the guidelines committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2017;23(11):1826–1838. - PubMed
-
- Horwitz SM, Ansell S, Ai WZ, et al. T-cell lymphomas, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(3):285–308. - PubMed
-
- d'Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093–3099. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

