Phase 1 randomized trials to assess safety, pharmacokinetics, and vaginal bleeding associated with use of extended duration dapivirine and levonorgestrel vaginal rings
- PMID: 38838028
- PMCID: PMC11152307
- DOI: 10.1371/journal.pone.0304552
Phase 1 randomized trials to assess safety, pharmacokinetics, and vaginal bleeding associated with use of extended duration dapivirine and levonorgestrel vaginal rings
Abstract
Background: Vaginal rings formulated to deliver two drugs simultaneously have potential as user-controlled, long-acting methods for dual prevention of HIV and pregnancy.
Methods: Two phase 1 randomized trials (MTN-030/IPM 041 and MTN-044/IPM 053/CCN019) respectively enrolled 24 and 25 healthy, HIV-negative participants to evaluate safety, pharmacokinetics, and vaginal bleeding associated with use of a vaginal ring containing 200mg dapivirine (DPV) and 320mg levonorgestrel (LNG) designed for 90-day use. MTN-030/IPM 041 compared the DPV/LNG ring to a DPV-only ring (200mg) over 14 days of use. MTN-044/IPM 053/CCN019 compared continuous or cyclic use of the DPV/LNG ring over 90 days of use. Safety was assessed by recording adverse events (AEs). DPV and LNG concentrations were quantified in plasma, cervicovaginal fluid, and cervical tissue. Vaginal bleeding was self-reported.
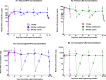
Results: There were no differences in the proportion of participants with grade ≥2 genitourinary AEs or grade ≥3 AEs with DPV/LNG ring vs. DPV ring use (p = .22), or with DPV/LNG ring continuous vs. cyclic use (p = .67). Higher plasma DPV concentrations were observed in users of DPV/LNG compared to DPV-only rings (Cmax p = 0.049; AUC p = 0.091). Plasma DPV and LNG concentrations were comparable with continuous and cyclic use (Cmax p = 0.74; AUC p = 0.25). With cyclic use, median nadir plasma DPV concentration was approximately 300 pg/mL two days after removal and median t1/2 for cervicovaginal fluid DPV concentration was 5.76 hours (n = 3). Overall bleeding experiences did not differ between continuous and cyclic users (p = 0.12).
Conclusions: The extended duration DPV/ LNG rings were well tolerated and the observed DPV concentrations in plasma and cervicovaginal fluid when used continuously exceeded concentrations observed in previous DPV ring efficacy studies. LNG concentrations in plasma were comparable with other efficacious LNG-based contraceptives. Genital DPV concentrations had a short half-life and were thus not well sustained following ring removal.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The following authors have read the journal’s policy and have competing interests: Sharon L. Achilles has received consulting fees from Mayne Pharma and Merck and has received research funding from The National Institutes of Health, the US Food and Drug Administration, the Pennsylvania Department of Health, Society of Family Planning Research Fund, Estetra SRL (an affiliate company of Mithra Pharmaceuticals), EvoFem, and Merck, all of which were managed by Magee-Womens Research Institute. Barbra A. Richardson has received payment from Gilead Sciences for DSMB membership. Brid Devlin and John Steytler were full-time salaried employees of the International Partnership for Microbicides (IPM), a non-profit company registered in the United States of America, at the time the work was performed. Craig W. Hendrix is an Inventor on patent relating to vaginal microbicides and the founder of a microbicide development company, both unrelated to this study product and both managed by Johns Hopkins University. Beatrice A. Chen has served on a Merck & Co. advisory board and has received research grants from Sebela, Mylan, and Medicines360, all of which were managed by Magee-Womens Research Institute. NIH employees (Diana L. Blithe, Jill Brown, and Jeanna M. Piper) contributed to the study design, manuscript development and the decision to publish as well as providing safety oversight during study conduct but had no role in data collection and analysis. All other authors have declared that no competing interests exist. This does not alter our adherence to PLOS One policies on sharing data and materials.
Figures
Similar articles
-
Acceptability of a dapivirine levonorgestrel vaginal ring in two Phase 1 trials (MTN-030/IPM 041 and MTN-044/IPM 053/CCN019): Implications for multipurpose prevention technology development.PLoS One. 2025 Jan 14;20(1):e0312957. doi: 10.1371/journal.pone.0312957. eCollection 2025. PLoS One. 2025. PMID: 39808648 Free PMC article. Clinical Trial.
-
Phase 1 pharmacokinetics and safety study of extended duration dapivirine vaginal rings in the United States.J Int AIDS Soc. 2021 Jun;24(6):e25747. doi: 10.1002/jia2.25747. J Int AIDS Soc. 2021. PMID: 34118115 Free PMC article. Clinical Trial.
-
Phase 2a Safety, Pharmacokinetics, and Acceptability of Dapivirine Vaginal Rings in US Postmenopausal Women.Clin Infect Dis. 2019 Mar 19;68(7):1144-1151. doi: 10.1093/cid/ciy654. Clin Infect Dis. 2019. PMID: 30289485 Free PMC article. Clinical Trial.
-
Skin patch and vaginal ring versus combined oral contraceptives for contraception.Cochrane Database Syst Rev. 2013 Apr 30;2013(4):CD003552. doi: 10.1002/14651858.CD003552.pub4. Cochrane Database Syst Rev. 2013. PMID: 23633314 Free PMC article. Review.
-
Levonorgestrel intrauterine system for endometrial protection in women with breast cancer on adjuvant tamoxifen.Cochrane Database Syst Rev. 2015 Dec 9;2015(12):CD007245. doi: 10.1002/14651858.CD007245.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2020 Dec 21;12:CD007245. doi: 10.1002/14651858.CD007245.pub4 PMID: 26649916 Free PMC article. Updated. Review.
References
-
- Slaymaker E, Scott RH, Palmer MJ, Palla L, Marston M, Gonsalves L, et al.. Trends in sexual activity and demand for and use of modern contraceptive methods in 74 countries: a retrospective analysis of nationally representative surveys. Lancet Glob Health. 2020. Apr;8(4): e567–79. doi: 10.1016/S2214-109X(20)30060-7 - DOI - PMC - PubMed
-
- United Nations. Contraceptive Use by Method 2019: Data Booklet [Internet]. UN; 2019. [cited 2021 Apr 29]. Available from: https://www.un-ilibrary.org/content/books/9789210046527.
-
- Bearak J, Popinchalk A, Ganatra B, Moller AB, Tunçalp Ö, Beavin C, et al.. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990–2019. Lancet Glob Health. 2020. Sep;8(9): e1152–61. doi: 10.1016/S2214-109X(20)30315-6 - DOI - PubMed
-
- UNAIDS_FactSheet_en.pdf [Internet]. [cited 2021 Apr 29]. Available from: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_....
-
- Sexually transmitted infections (STIs) [Internet]. [cited 2021 Apr 29]. Available from: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-in....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

