Sex-related immunity: could Toll-like receptors be the answer in acute inflammatory response?
- PMID: 38835761
- PMCID: PMC11148260
- DOI: 10.3389/fimmu.2024.1379754
Sex-related immunity: could Toll-like receptors be the answer in acute inflammatory response?
Abstract
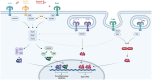
An increasing number of studies have highlighted the existence of a sex-specific immune response, wherein men experience a worse prognosis in cases of acute inflammatory diseases. Initially, this sex-dependent inflammatory response was attributed to the influence of sex hormones. However, a growing body of evidence has shifted the focus toward the influence of chromosomes rather than sex hormones in shaping these inflammatory sex disparities. Notably, certain pattern recognition receptors, such as Toll-like receptors (TLRs), and their associated immune pathways have been implicated in driving the sex-specific immune response. These receptors are encoded by genes located on the X chromosome. TLRs are pivotal components of the innate immune system, playing crucial roles in responding to infectious diseases, including bacterial and viral pathogens, as well as trauma-related conditions. Importantly, the TLR-mediated inflammatory responses, as indicated by the production of specific proteins and cytokines, exhibit discernible sex-dependent patterns. In this review, we delve into the subject of sex bias in TLR activation and explore its clinical implications relatively to both the X chromosome and the hormonal environment. The overarching objective is to enhance our understanding of the fundamental mechanisms underlying these sex differences.
Keywords: TLR-Toll-like receptor; X chromosome; hormones; infection; innate immunity; sepsis; sex; trauma.
Copyright © 2024 Popotas, Casimir, Corazza and Lefèvre.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Differential Effects of Toll-Like Receptor Signaling on the Activation of Immune Responses in the Upper Respiratory Tract.Microbiol Spectr. 2022 Feb 23;10(1):e0114421. doi: 10.1128/spectrum.01144-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196817 Free PMC article.
-
Targeting the Toll-like receptor pathway as a therapeutic strategy for neonatal infection.Am J Physiol Regul Integr Comp Physiol. 2021 Dec 1;321(6):R879-R902. doi: 10.1152/ajpregu.00307.2020. Epub 2021 Oct 6. Am J Physiol Regul Integr Comp Physiol. 2021. PMID: 34612068 Review.
-
Translational mini-review series on Toll-like receptors: networks regulated by Toll-like receptors mediate innate and adaptive immunity.Clin Exp Immunol. 2007 Feb;147(2):199-207. doi: 10.1111/j.1365-2249.2006.03203.x. Clin Exp Immunol. 2007. PMID: 17223959 Free PMC article. Review.
-
Toll-like receptors and their crosstalk with other innate receptors in infection and immunity.Immunity. 2011 May 27;34(5):637-50. doi: 10.1016/j.immuni.2011.05.006. Immunity. 2011. PMID: 21616434 Review.
-
Direct and indirect role of Toll-like receptors in T cell mediated immunity.Cell Mol Immunol. 2004 Aug;1(4):239-46. Cell Mol Immunol. 2004. PMID: 16225766 Review.
Cited by
-
Serum from patients with cirrhosis undergoing liver transplantation induces permeability in human pulmonary microvascular endothelial cells ex vivo.Front Med (Lausanne). 2024 Jul 3;11:1412891. doi: 10.3389/fmed.2024.1412891. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39021821 Free PMC article.
-
Identification biomarkers in disease progression of obstructive sleep apnea from children serum based on WGCNA and Mfuzz.Front Neurol. 2024 Oct 1;15:1452507. doi: 10.3389/fneur.2024.1452507. eCollection 2024. Front Neurol. 2024. PMID: 39410993 Free PMC article.
-
Sex Disparities in Staphylococcus aureus Bacteremia Mortality.JAMA Netw Open. 2024 Oct 1;7(10):e2441502. doi: 10.1001/jamanetworkopen.2024.41502. JAMA Netw Open. 2024. PMID: 39476238 Free PMC article.
References
-
- Vahidy FS, Pan AP, Ahnstedt H, Munshi Y, Choi HA, Tiruneh Y, et al. . Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: Cross-sectional analysis from a diverse US metropolitan area. PloS One. (2021) 16:e0245556. doi: 10.1371/journal.pone.0245556 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

