COVID-19 Vaccine Effectiveness and Digital Pandemic Surveillance in Germany (eCOV Study): Web Application-Based Prospective Observational Cohort Study
- PMID: 38833299
- PMCID: PMC11185909
- DOI: 10.2196/47070
COVID-19 Vaccine Effectiveness and Digital Pandemic Surveillance in Germany (eCOV Study): Web Application-Based Prospective Observational Cohort Study
Abstract
Background: The COVID-19 pandemic posed significant challenges to global health systems. Efficient public health responses required a rapid and secure collection of health data to improve the understanding of SARS-CoV-2 and examine the vaccine effectiveness (VE) and drug safety of the novel COVID-19 vaccines.
Objective: This study (COVID-19 study on vaccinated and unvaccinated subjects over 16 years; eCOV study) aims to (1) evaluate the real-world effectiveness of COVID-19 vaccines through a digital participatory surveillance tool and (2) assess the potential of self-reported data for monitoring key parameters of the COVID-19 pandemic in Germany.
Methods: Using a digital study web application, we collected self-reported data between May 1, 2021, and August 1, 2022, to assess VE, test positivity rates, COVID-19 incidence rates, and adverse events after COVID-19 vaccination. Our primary outcome measure was the VE of SARS-CoV-2 vaccines against laboratory-confirmed SARS-CoV-2 infection. The secondary outcome measures included VE against hospitalization and across different SARS-CoV-2 variants, adverse events after vaccination, and symptoms during infection. Logistic regression models adjusted for confounders were used to estimate VE 4 to 48 weeks after the primary vaccination series and after third-dose vaccination. Unvaccinated participants were compared with age- and gender-matched participants who had received 2 doses of BNT162b2 (Pfizer-BioNTech) and those who had received 3 doses of BNT162b2 and were not infected before the last vaccination. To assess the potential of self-reported digital data, the data were compared with official data from public health authorities.
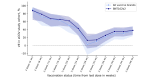
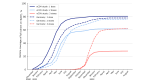
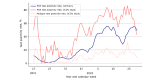
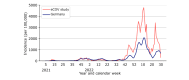
Results: We enrolled 10,077 participants (aged ≥16 y) who contributed 44,786 tests and 5530 symptoms. In this young, primarily female, and digital-literate cohort, VE against infections of any severity waned from 91.2% (95% CI 70.4%-97.4%) at week 4 to 37.2% (95% CI 23.5%-48.5%) at week 48 after the second dose of BNT162b2. A third dose of BNT162b2 increased VE to 67.6% (95% CI 50.3%-78.8%) after 4 weeks. The low number of reported hospitalizations limited our ability to calculate VE against hospitalization. Adverse events after vaccination were consistent with previously published research. Seven-day incidences and test positivity rates reflected the course of the pandemic in Germany when compared with official numbers from the national infectious disease surveillance system.
Conclusions: Our data indicate that COVID-19 vaccinations are safe and effective, and third-dose vaccinations partially restore protection against SARS-CoV-2 infection. The study showcased the successful use of a digital study web application for COVID-19 surveillance and continuous monitoring of VE in Germany, highlighting its potential to accelerate public health decision-making. Addressing biases in digital data collection is vital to ensure the accuracy and reliability of digital solutions as public health tools.
Keywords: BNT162b2; COVID-19; COVID-19 vaccines; Germany; SARS-CoV-2; data collection; digital public health; disease surveillance; effectiveness; participatory disease surveillance; tool; vaccination; vaccine effectiveness; web application.
©Anna-Lena Lang, Nils Hohmuth, Vukašin Višković, Stefan Konigorski, Stefan Scholz, Felix Balzer, Cornelius Remschmidt, Rasmus Leistner. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 04.06.2024.
Conflict of interest statement
Conflicts of Interest: ALL, NH, CR, and VV worked for Data4Life, the company that developed the study and study app as well as the underlying research infrastructure. Outside the submitted work, FB reports grants from the German Federal Ministry of Education and Research, grants from the German Federal Ministry of Health, grants from the Berlin Institute of Health, personal fees from Medtronic and Elsevier Publishing, grants from the Hans Böckler Foundation, and travel support from the Robert Koch Institute. All other authors declare no other conflicts of interest.
Figures
Similar articles
-
Effectiveness of a Third Dose of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance - VISION Network, 10 States, August 2021-January 2022.MMWR Morb Mortal Wkly Rep. 2022 Jan 21;71(4):139-145. doi: 10.15585/mmwr.mm7104e3. MMWR Morb Mortal Wkly Rep. 2022. PMID: 35085224 Free PMC article.
-
Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data.Lancet. 2021 May 15;397(10287):1819-1829. doi: 10.1016/S0140-6736(21)00947-8. Epub 2021 May 5. Lancet. 2021. PMID: 33964222 Free PMC article.
-
Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study.Lancet Infect Dis. 2022 Jan;22(1):56-63. doi: 10.1016/S1473-3099(21)00479-5. Epub 2021 Sep 9. Lancet Infect Dis. 2022. PMID: 34509185 Free PMC article.
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article. Review.
-
Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis.Infect Dis Poverty. 2021 Nov 14;10(1):132. doi: 10.1186/s40249-021-00915-3. Infect Dis Poverty. 2021. PMID: 34776011 Free PMC article. Review.
References
-
- Kennedy B, Fitipaldi H, Hammar U, Maziarz M, Tsereteli N, Oskolkov N, Varotsis G, Franks CA, Nguyen D, Spiliopoulos L, Adami H, Björk J, Engblom S, Fall K, Grimby-Ekman A, Litton J, Martinell M, Oudin A, Sjöström T, Timpka T, Sudre CH, Graham MS, du Cadet JL, Chan AT, Davies R, Ganesh S, May A, Ourselin S, Pujol JC, Selvachandran S, Wolf J, Spector TD, Steves CJ, Gomez MF, Franks PW, Fall T. App-based COVID-19 syndromic surveillance and prediction of hospital admissions in COVID symptom study Sweden. Nat Commun. 2022 Apr 21;13(1):2110. doi: 10.1038/s41467-022-29608-7. doi: 10.1038/s41467-022-29608-7.10.1038/s41467-022-29608-7 - DOI - DOI - PMC - PubMed
-
- Menni C, May A, Polidori L, Louca P, Wolf J, Capdevila J, Hu C, Ourselin S, Steves CJ, Valdes AM, Spector TD. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis. 2022 Jul;22(7):1002–10. doi: 10.1016/S1473-3099(22)00146-3. https://linkinghub.elsevier.com/retrieve/pii/S1473-3099(22)00146-3 S1473-3099(22)00146-3 - DOI - PMC - PubMed
-
- Liang F. COVID-19 and health code: how digital platforms tackle the pandemic in China. Soc Media Soc. 2020 Jul;6(3):2056305120947657. doi: 10.1177/2056305120947657. https://journals.sagepub.com/doi/abs/10.1177/2056305120947657?url_ver=Z3... 10.1177_2056305120947657 - DOI - DOI - PMC - PubMed
-
- Corona data donation project. Robert Koch Institute & Research on Complex Systems of Humboldt University of Berlin. [2022-12-12]. https://corona-datenspende.de/science/en .
-
- Shapiro A, Marinsek N, Clay I, Bradshaw B, Ramirez E, Min J, Trister A, Wang Y, Althoff T, Foschini L. Characterizing COVID-19 and influenza illnesses in the real world via person-generated health data. Patterns (N Y) 2021 Jan 08;2(1):100188. doi: 10.1016/j.patter.2020.100188. https://linkinghub.elsevier.com/retrieve/pii/S2666-3899(20)30258-0 S2666-3899(20)30258-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

