Gas-propelled nanomotors alleviate colitis through the regulation of intestinal immunoenvironment-hematopexis-microbiota circuits
- PMID: 38828144
- PMCID: PMC11143748
- DOI: 10.1016/j.apsb.2024.02.008
Gas-propelled nanomotors alleviate colitis through the regulation of intestinal immunoenvironment-hematopexis-microbiota circuits
Erratum in
-
Erratum: Author correction to "Gas-propelled nanomotors alleviate colitis through the regulation of intestinal immunoenvironment-hematopexis-microbiota circuits" [Acta Pharm Sin B 14 (2024) 2732-2747].Acta Pharm Sin B. 2024 Sep;14(9):4193. doi: 10.1016/j.apsb.2024.06.005. Epub 2024 Jun 15. Acta Pharm Sin B. 2024. PMID: 39309506 Free PMC article.
Abstract
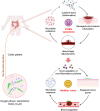
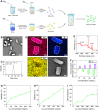
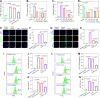
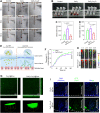
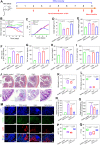
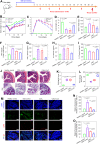
The progression of ulcerative colitis (UC) is associated with immunologic derangement, intestinal hemorrhage, and microbiota imbalance. While traditional medications mainly focus on mitigating inflammation, it remains challenging to address multiple symptoms. Here, a versatile gas-propelled nanomotor was constructed by mild fusion of post-ultrasonic CaO2 nanospheres with Cu2O nanoblocks. The resulting CaO2-Cu2O possessed a desirable diameter (291.3 nm) and a uniform size distribution. It could be efficiently internalized by colonic epithelial cells and macrophages, scavenge intracellular reactive oxygen/nitrogen species, and alleviate immune reactions by pro-polarizing macrophages to the anti-inflammatory M2 phenotype. This nanomotor was found to penetrate through the mucus barrier and accumulate in the colitis mucosa due to the driving force of the generated oxygen bubbles. Rectal administration of CaO2-Cu2O could stanch the bleeding, repair the disrupted colonic epithelial layer, and reduce the inflammatory responses through its interaction with the genes relevant to blood coagulation, anti-oxidation, wound healing, and anti-inflammation. Impressively, it restored intestinal microbiota balance by elevating the proportions of beneficial bacteria (e.g., Odoribacter and Bifidobacterium) and decreasing the abundances of harmful bacteria (e.g., Prevotellaceae and Helicobacter). Our gas-driven CaO2-Cu2O offers a promising therapeutic platform for robust treatment of UC via the rectal route.
Keywords: Anti-inflammation; Blood coagulation; Hematopexis; Immune regulation; Microbiota rebalance; Nanomotor; Rectal administration; Ulcerative colitis.
© 2024 The Authors.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Erratum: Author correction to "Gas-propelled nanomotors alleviate colitis through the regulation of intestinal immunoenvironment-hematopexis-microbiota circuits" [Acta Pharm Sin B 14 (2024) 2732-2747].Acta Pharm Sin B. 2024 Sep;14(9):4193. doi: 10.1016/j.apsb.2024.06.005. Epub 2024 Jun 15. Acta Pharm Sin B. 2024. PMID: 39309506 Free PMC article.
-
Transient Mild Photothermia Improves Therapeutic Performance of Oral Nanomedicines with Enhanced Accumulation in the Colitis Mucosa.Adv Mater. 2024 Apr;36(14):e2309516. doi: 10.1002/adma.202309516. Epub 2024 Jan 10. Adv Mater. 2024. PMID: 38085512
-
Protective effect of synbiotic combination of Lactobacillus plantarum SC-5 and olive oil extract tyrosol in a murine model of ulcerative colitis.J Transl Med. 2024 Mar 25;22(1):308. doi: 10.1186/s12967-024-05026-9. J Transl Med. 2024. PMID: 38528541 Free PMC article.
-
Traditional Chinese Medicine: A promising strategy to regulate the imbalance of bacterial flora, impaired intestinal barrier and immune function attributed to ulcerative colitis through intestinal microecology.J Ethnopharmacol. 2024 Jan 10;318(Pt A):116879. doi: 10.1016/j.jep.2023.116879. Epub 2023 Jul 5. J Ethnopharmacol. 2024. PMID: 37419224 Review.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
References
-
- Doherty G., Katsanos K.H., Burisch J., Allez M., Papamichael K., Stallmach A., et al. European crohn's and colitis organisation topical review on treatment withdrawal ‘exit strategies’ in inflammatory bowel disease. J Crohns Colitis. 2018;12:17–31. - PubMed
-
- Du L., Ha C. Epidemiology and pathsssogenesis of ulcerative colitis. Gastroenterol Clin. 2020;49:643–654. - PubMed
-
- Shanahan F. Pathogenesis of ulcerative colitis. Lancet. 1993;342:407–411. - PubMed
LinkOut - more resources
Full Text Sources

