GNAS mutations suppress cell invasion by activating MEG3 in growth hormone-secreting pituitary adenoma
- PMID: 38827318
- PMCID: PMC11136687
- DOI: 10.32604/or.2024.046007
GNAS mutations suppress cell invasion by activating MEG3 in growth hormone-secreting pituitary adenoma
Abstract
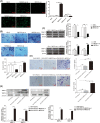
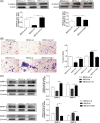
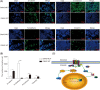
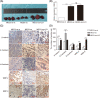
Approximately 30%-40% of growth hormone-secreting pituitary adenomas (GHPAs) harbor somatic activating mutations in GNAS (α subunit of stimulatory G protein). Mutations in GNAS are associated with clinical features of smaller and less invasive tumors. However, the role of GNAS mutations in the invasiveness of GHPAs is unclear. GNAS mutations were detected in GHPAs using a standard polymerase chain reaction (PCR) sequencing procedure. The expression of mutation-associated maternally expressed gene 3 (MEG3) was evaluated with RT-qPCR. MEG3 was manipulated in GH3 cells using a lentiviral expression system. Cell invasion ability was measured using a Transwell assay, and epithelial-mesenchymal transition (EMT)-associated proteins were quantified by immunofluorescence and western blotting. Finally, a tumor cell xenograft mouse model was used to verify the effect of MEG3 on tumor growth and invasiveness. The invasiveness of GHPAs was significantly decreased in mice with mutated GNAS compared with that in mice with wild-type GNAS. Consistently, the invasiveness of mutant GNAS-expressing GH3 cells decreased. MEG3 is uniquely expressed at high levels in GHPAs harboring mutated GNAS. Accordingly, MEG3 upregulation inhibited tumor cell invasion, and conversely, MEG3 downregulation increased tumor cell invasion. Mechanistically, GNAS mutations inhibit EMT in GHPAs. MEG3 in mutated GNAS cells prevented cell invasion through the inactivation of the Wnt/β-catenin signaling pathway, which was further validated in vivo. Our data suggest that GNAS mutations may suppress cell invasion in GHPAs by regulating EMT through the activation of the MEG3/Wnt/β-catenin signaling pathway.
Keywords: EMT; GHPAs; GNAS mutation; MEG3; β-catenin.
© 2024 Tang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest to report regarding the present study.
Figures



Similar articles
-
Analysis of GNAS mutations in 60 growth hormone secreting pituitary tumors: correlation with clinical and pathological characteristics and surgical outcome based on highly sensitive GH and IGF-I criteria for remission.Pituitary. 2007;10(3):275-82. doi: 10.1007/s11102-007-0058-2. Pituitary. 2007. PMID: 17594522
-
GNAS promotes inflammation-related hepatocellular carcinoma progression by promoting STAT3 activation.Cell Mol Biol Lett. 2020 Feb 24;25:8. doi: 10.1186/s11658-020-00204-1. eCollection 2020. Cell Mol Biol Lett. 2020. PMID: 32123532 Free PMC article.
-
Long non-coding RNA GNAS-AS1 promotes cell migration and invasion via regulating Wnt/β-catenin pathway in nasopharyngeal carcinoma.Eur Rev Med Pharmacol Sci. 2020 Mar;24(6):3077-3084. doi: 10.26355/eurrev_202003_20672. Eur Rev Med Pharmacol Sci. 2020. PMID: 32271425
-
GH/PRL-secreting pituitary macroadenoma associated with GNAS p.Gln227Leu mutation: pediatric case report and review.Endocr J. 2019 May 28;66(5):403-408. doi: 10.1507/endocrj.EJ18-0370. Epub 2019 Mar 28. Endocr J. 2019. PMID: 30814395 Review.
-
GNAS imprinting and pituitary tumors.Mol Cell Endocrinol. 2010 Sep 15;326(1-2):15-8. doi: 10.1016/j.mce.2010.04.009. Epub 2010 Apr 14. Mol Cell Endocrinol. 2010. PMID: 20398730 Review.
References
-
- Park, H. H., Kim, E. H., Ku, C. R., Lee, E. J., Kim, S. H. (2018). Outcomes of aggressive surgical resection in growth hormone-secreting pituitary adenomas with cavernous sinus invasion. World Neurosurgery , 117, e280−e9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
