This is a preprint.
Murine modeling of menstruation identifies immune correlates of protection during Chlamydia muridarum challenge
- PMID: 38826233
- PMCID: PMC11142139
- DOI: 10.1101/2024.05.21.595090
Murine modeling of menstruation identifies immune correlates of protection during Chlamydia muridarum challenge
Abstract
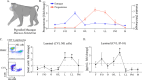
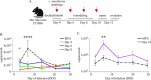
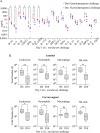
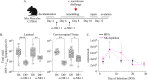
The menstrual cycle influences the risk of acquiring sexually transmitted infections (STIs), including Chlamydia trachomatis (C. trachomatis), although the underlying immune contributions are poorly defined. A mouse model simulating the immune-mediated process of menstruation could provide valuable insights into tissue-specific determinants of protection against chlamydial infection within the cervicovaginal and uterine mucosae comprising the female reproductive tract (FRT). Here, we used the pseudopregnancy approach in naïve C57Bl/6 mice and performed vaginal challenge with Chlamydia muridarum (C. muridarum) at decidualization, endometrial tissue remodeling, or uterine repair. This strategy identified that the time frame comprising uterine repair correlated with robust infection and greater bacterial burden as compared with mice on hormonal contraception, while challenges during endometrial remodeling were least likely to result in a productive infection. By comparing the infection site at early time points following chlamydial challenge, we found that a greater abundance of innate effector populations and proinflammatory signaling, including IFNγ correlated with protection. FRT immune profiling in uninfected mice over pseudopregnancy or in pig-tailed macaques over the menstrual cycle identified NK cell infiltration into the cervicovaginal tissues and lumen over the course of endometrial remodeling. Notably, NK cell depletion over this time frame reversed protection, with mice now productively infected with C. muridarum following challenge. This study shows that the pseudopregnancy murine menstruation model recapitulates immune changes in the FRT as a result of endometrial remodeling and identifies NK cell localization at the FRT as essential for immune protection against primary C. muridarum infection.
Figures


Similar articles
-
Chlamydia-Specific IgA Secretion in the Female Reproductive Tract Induced via Per-Oral Immunization Confers Protection against Primary Chlamydia Challenge.Infect Immun. 2020 Dec 15;89(1):e00413-20. doi: 10.1128/IAI.00413-20. Print 2020 Dec 15. Infect Immun. 2020. PMID: 33139380 Free PMC article.
-
Neisseria gonorrhoeae Coinfection during Chlamydia muridarum Genital Latency Does Not Modulate Murine Vaginal Bacterial Shedding.Microbiol Spectr. 2023 Jun 15;11(3):e0450022. doi: 10.1128/spectrum.04500-22. Epub 2023 Apr 11. Microbiol Spectr. 2023. PMID: 37039695 Free PMC article.
-
Intranasal vaccination with Chlamydia pneumoniae induces cross-species immunity against genital Chlamydia muridarum challenge in mice.PLoS One. 2013 May 31;8(5):e64917. doi: 10.1371/journal.pone.0064917. Print 2013. PLoS One. 2013. PMID: 23741420 Free PMC article.
-
IFNγ and Antibody Synergize To Enhance Protective Immunity against Chlamydia Dissemination and Female Reproductive Tract Reinfections.Infect Immun. 2022 Dec 15;90(12):e0032822. doi: 10.1128/iai.00328-22. Epub 2022 Nov 14. Infect Immun. 2022. PMID: 36374101 Free PMC article.
-
Chlamydia Spreading from the Genital Tract to the Gastrointestinal Tract - A Two-Hit Hypothesis.Trends Microbiol. 2018 Jul;26(7):611-623. doi: 10.1016/j.tim.2017.12.002. Epub 2017 Dec 27. Trends Microbiol. 2018. PMID: 29289422 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
