Bioisostere-conjugated fluorescent probes for live-cell protein imaging without non-specific organelle accumulation
- PMID: 38817570
- PMCID: PMC11134342
- DOI: 10.1039/d3sc06957e
Bioisostere-conjugated fluorescent probes for live-cell protein imaging without non-specific organelle accumulation
Abstract
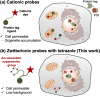
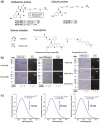
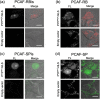
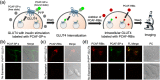
Specific labeling of proteins using membrane-permeable fluorescent probes is a powerful technique for bioimaging. Cationic fluorescent dyes with high fluorescence quantum yield, photostability, and water solubility provide highly useful scaffolds for protein-labeling probes. However, cationic probes generally show undesired accumulation in organelles, which causes a false-positive signal in localization analysis. Herein, we report a design strategy for probes that suppress undesired organelle accumulation using a bioisostere for intracellular protein imaging in living cells. Our design allows the protein labeling probes to possess both membrane permeability and suppress non-specific accumulation and has been shown to use several protein labeling systems, such as PYP-tag and Halo tag systems. We further developed a fluorogenic PYP-tag labeling probe for intracellular proteins and used it to visualize multiple localizations of target proteins in the intracellular system. Our strategy offers a versatile design for undesired accumulation-suppressed probes with cationic dye scaffolds and provides a valuable tool for intracellular protein imaging.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Development of fluorogenic probes for quick no-wash live-cell imaging of intracellular proteins.J Am Chem Soc. 2013 Aug 21;135(33):12360-5. doi: 10.1021/ja405745v. Epub 2013 Aug 8. J Am Chem Soc. 2013. PMID: 23927377
-
Development of an effective protein-labeling system based on smart fluorogenic probes.J Biol Inorg Chem. 2019 Jun;24(4):443-455. doi: 10.1007/s00775-019-01669-y. Epub 2019 May 31. J Biol Inorg Chem. 2019. PMID: 31152238 Review.
-
Development of Fluorogenic Probes for Rapid High-Contrast Imaging of Transient Nuclear Localization of Sirtuin 3.Chembiochem. 2020 Mar 2;21(5):656-662. doi: 10.1002/cbic.201900568. Epub 2019 Nov 4. Chembiochem. 2020. PMID: 31518474
-
Small-molecule-based protein-labeling technology in live cell studies: probe-design concepts and applications.Acc Chem Res. 2014 Jan 21;47(1):247-56. doi: 10.1021/ar400135f. Epub 2013 Aug 8. Acc Chem Res. 2014. PMID: 23927788 Review.
-
Redesign of a Fluorogenic Labeling System To Improve Surface Charge, Brightness, and Binding Kinetics for Imaging the Functional Localization of Bromodomains.Angew Chem Int Ed Engl. 2015 Nov 23;54(48):14368-71. doi: 10.1002/anie.201506935. Epub 2015 Oct 5. Angew Chem Int Ed Engl. 2015. PMID: 26434386
Cited by
-
Empowering brain tumor management: chimeric antigen receptor macrophage therapy.Theranostics. 2024 Sep 3;14(14):5725-5742. doi: 10.7150/thno.98290. eCollection 2024. Theranostics. 2024. PMID: 39310093 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

