Factors influencing the participation of pregnant and lactating women in clinical trials: A mixed-methods systematic review
- PMID: 38814991
- PMCID: PMC11139290
- DOI: 10.1371/journal.pmed.1004405
Factors influencing the participation of pregnant and lactating women in clinical trials: A mixed-methods systematic review
Abstract
Background: Poor representation of pregnant and lactating women and people in clinical trials has marginalised their health concerns and denied the maternal-fetal/infant dyad benefits of innovation in therapeutic research and development. This mixed-methods systematic review synthesised factors affecting the participation of pregnant and lactating women in clinical trials, across all levels of the research ecosystem.
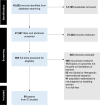
Methods and findings: We searched 8 databases from inception to 14 February 2024 to identify qualitative, quantitative, and mixed-methods studies that described factors affecting participation of pregnant and lactating women in vaccine and therapeutic clinical trials in any setting. We used thematic synthesis to analyse the qualitative literature and assessed confidence in each qualitative review finding using the GRADE-CERQual approach. We compared quantitative data against the thematic synthesis findings to assess areas of convergence or divergence. We mapped review findings to the Theoretical Domains Framework (TDF) and Capability, Opportunity, and Motivation Model of Behaviour (COM-B) to inform future development of behaviour change strategies. We included 60 papers from 27 countries. We grouped 24 review findings under 5 overarching themes: (a) interplay between perceived risks and benefits of participation in women's decision-making; (b) engagement between women and the medical and research ecosystems; (c) gender norms and decision-making autonomy; (d) factors affecting clinical trial recruitment; and (e) upstream factors in the research ecosystem. Women's willingness to participate in trials was affected by: perceived risk of the health condition weighed against an intervention's risks and benefits, therapeutic optimism, intervention acceptability, expectations of receiving higher quality care in a trial, altruistic motivations, intimate relationship dynamics, and power and trust in medicine and research. Health workers supported women's participation in trials when they perceived clinical equipoise, had hope for novel therapeutic applications, and were convinced an intervention was safe. For research staff, developing reciprocal relationships with health workers, having access to resources for trial implementation, ensuring the trial was visible to potential participants and health workers, implementing a woman-centred approach when communicating with potential participants, and emotional orientations towards the trial were factors perceived to affect recruitment. For study investigators and ethics committees, the complexities and subjectivities in risk assessments and trial design, and limited funding of such trials contributed to their reluctance in leading and approving such trials. All included studies focused on factors affecting participation of cisgender pregnant women in clinical trials; future research should consider other pregnancy-capable populations, including transgender and nonbinary people.
Conclusions: This systematic review highlights diverse factors across multiple levels and stakeholders affecting the participation of pregnant and lactating women in clinical trials. By linking identified factors to frameworks of behaviour change, we have developed theoretically informed strategies that can help optimise pregnant and lactating women's engagement, participation, and trust in such trials.
Copyright: © 2024 Shankar et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Understanding willingness and barriers to participate in clinical trials during pregnancy and lactation: findings from a US study.BMC Pregnancy Childbirth. 2024 Jul 26;24(1):504. doi: 10.1186/s12884-024-06710-w. BMC Pregnancy Childbirth. 2024. PMID: 39060985 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Provision and uptake of routine antenatal services: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2019 Jun 12;6(6):CD012392. doi: 10.1002/14651858.CD012392.pub2. Cochrane Database Syst Rev. 2019. PMID: 31194903 Free PMC article.
-
Perceptions and experiences of the prevention, detection, and management of postpartum haemorrhage: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2023 Nov 27;11(11):CD013795. doi: 10.1002/14651858.CD013795.pub2. Cochrane Database Syst Rev. 2023. PMID: 38009552 Free PMC article. Review.
-
Barriers to and facilitators of smoking cessation in pregnancy and following childbirth: literature review and qualitative study.Health Technol Assess. 2017 Jun;21(36):1-158. doi: 10.3310/hta21360. Health Technol Assess. 2017. PMID: 28661375 Free PMC article. Review.
Cited by
-
Eliminating gender bias in biomedical research requires fair inclusion of pregnant women and gender diverse people.Commun Med (Lond). 2024 Oct 23;4(1):211. doi: 10.1038/s43856-024-00629-1. Commun Med (Lond). 2024. PMID: 39443672 Free PMC article. Review.
-
Understanding willingness and barriers to participate in clinical trials during pregnancy and lactation: findings from a US study.BMC Pregnancy Childbirth. 2024 Jul 26;24(1):504. doi: 10.1186/s12884-024-06710-w. BMC Pregnancy Childbirth. 2024. PMID: 39060985 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical