Improving the Biocompatibility and Functionality of Neural Interface Devices with Silica Nanoparticles
- PMID: 38814586
- PMCID: PMC11191400
- DOI: 10.1021/acs.accounts.4c00160
Improving the Biocompatibility and Functionality of Neural Interface Devices with Silica Nanoparticles
Abstract
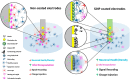
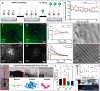
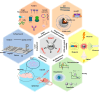
ConspectusNeural interface technologies enable bidirectional communication between the nervous system and external instrumentation. Advancements in neural interface devices not only open new frontiers for neuroscience research, but also hold great promise for clinical diagnosis, therapy, and rehabilitation for various neurological disorders. However, the performance of current neural electrode devices, often termed neural probes, is far from satisfactory. Glial scarring, neuronal degeneration, and electrode degradation eventually cause the devices to lose their connection with the brain. To improve the chronic performance of neural probes, efforts need to be made on two fronts: enhancing the physiochemical properties of the electrode materials and mitigating the undesired host tissue response.In this Account, we discuss our efforts in developing silica-nanoparticle-based (SiNP) coatings aimed at enhancing neural probe electrochemical properties and promoting device-tissue integration. Our work focuses on three approaches:(1) SiNPs' surface texturization to enhance biomimetic protein coatings for promoting neural integration. Through covalent immobilization, SiNP introduces biologically relevant nanotopography to neural probe surfaces, enhancing neuronal cell attachments and inhibiting microglia. The SiNP base coating further increases the binding density and stability of bioactive molecules such as L1CAM and facilitates the widespread dissemination of biomimetic coatings. (2) Doping SiNPs into conductive polymer electrode coatings improves the electrochemical properties and stability. As neural interface devices are moving to subcellular sizes to escape the immune response and high electrode site density to increase spatial resolution, the electrode sites need to be very small. The smaller electrode size comes at the cost of a high electrode impedance, elevated thermal noise, and insufficient charge injection capacity. Electrochemically deposited conductive polymer films reduce electrode impedance but do not endure prolonged electrical cycling. When incorporated into conductive polymer coatings as a dopant, the SiNP provides structural support for the polymer thin films, significantly increasing their stability and durability. Low interfacial impedance maintained by the conducting polymer/SiNP composite is critical for extended electrode longevity and effective charge injection in chronic neural stimulation applications. (3) Porous nanoparticles are used as drug carriers in conductive polymer coatings for local drug/neurochemical delivery. When triggered by external electrical stimuli, drug molecules and neurochemicals can be released in a controlled manner. Such precise focal manipulation of cellular and vascular behavior enables us to probe brain circuitry and develop therapeutic applications.We foresee tremendous opportunities for further advancing the functionality of SiNP coatings by incorporating new nanoscale components and integrating the coating with other design strategies. With an enriched nanoscale toolbox and optimized design strategies, we can create customizable multifunctional and multimodal neural interfaces that can operate at multiple spatial levels and seamlessly integrate with the host tissue for extended applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Surface modification of neural recording electrodes with conducting polymer/biomolecule blends.J Biomed Mater Res. 2001 Aug;56(2):261-72. doi: 10.1002/1097-4636(200108)56:2<261::aid-jbm1094>3.0.co;2-i. J Biomed Mater Res. 2001. PMID: 11340598
-
Soft, Fuzzy, and Bioactive Conducting Polymers for Improving the Chronic Performance of Neural Prosthetic Devices.In: Reichert WM, editor. Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment. Boca Raton (FL): CRC Press/Taylor & Francis; 2008. Chapter 7. In: Reichert WM, editor. Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment. Boca Raton (FL): CRC Press/Taylor & Francis; 2008. Chapter 7. PMID: 21204405 Free Books & Documents. Review.
-
Improving Deep Brain Stimulation Electrode Performance in vivo Through Use of Conductive Hydrogel Coatings.Front Neurosci. 2021 Nov 5;15:761525. doi: 10.3389/fnins.2021.761525. eCollection 2021. Front Neurosci. 2021. PMID: 34803592 Free PMC article.
-
Fungal spore adhesion on glycidoxypropyltrimethoxy silane modified silica nanoparticle surfaces as revealed by single cell force spectroscopy.Biointerphases. 2020 Jun 17;15(3):031012. doi: 10.1116/6.0000142. Biointerphases. 2020. PMID: 32551719
-
Conducting polymers for neural interfaces: challenges in developing an effective long-term implant.Biomaterials. 2008 Aug-Sep;29(24-25):3393-9. doi: 10.1016/j.biomaterials.2008.04.047. Epub 2008 May 23. Biomaterials. 2008. PMID: 18501423 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

