Budesonide/Glycopyrrolate/Formoterol for the Management of COPD in a UK Primary Care Population: Real-World Use and Early Medication Success
- PMID: 38813078
- PMCID: PMC11134059
- DOI: 10.2147/COPD.S452624
Budesonide/Glycopyrrolate/Formoterol for the Management of COPD in a UK Primary Care Population: Real-World Use and Early Medication Success
Abstract
Purpose: Real-life research is needed to evaluate the effectiveness of budesonide/glycopyrrolate/formoterol (BGF) in routine COPD primary care management. We assessed the frequency of medication success among patients with COPD who initiated BGF using real-world data.
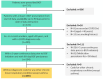
Patients and methods: Patients with a recorded diagnostic COPD code who started BGF with ≥2 prescriptions within 90-days were identified in the UK Optimum Patient Care Research Database and followed from first prescription until censoring at the end of follow-up (180-days), death, leaving database or end of data at 24/10/2022. The primary outcome was medication success at 90-days post-BGF initiation, defined as no major cardiac or respiratory event (ie no complicated COPD exacerbation, hospitalization for any respiratory event, myocardial infarction, new/hospitalized heart failure, and death) and no incidence of pneumonia. Medication success was also assessed at 180-days post-BGF initiation. Overall real-life medication success was claimed if the lower 95% confidence interval (CI) for the proportion of patients meeting the primary outcome was ≥70% (defined a priori).
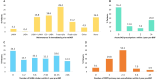
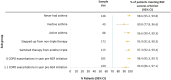
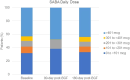
Results: Two hundred eighty-five patients were included. Prior to BGF initiation, these patients often had severe airflow obstruction (mean ppFEV1: 54.5%), were highly symptomatic (mMRC ≥2: 77.9% (n = 205/263); mean CAT score: 21.7 (SD 7.8)), with evidence of short-acting β2-agonist (SABA) over-use (≥3 inhalers/year: 62.1%, n=179/285), repeat OCS prescriptions (≥2 courses/year: 33.0%, n = 95/285) and multiple primary care consultations (≥2 visits/year: 61.1%, n = 174/285). Overall, 39.6% of patients (n = 113/285) switched from previous triple therapies. Real-life medication success was achieved by 96.5% of patients (n = 275/285 [95% CI: 93.6, 98.3]) during 90-days treatment with BGF and by 91.8% (n = 169/184 [95% CI: 86.9, 95.4]) of patients at 180-days. The prescribed daily dose of SABA remained stable over the study period.
Conclusion: The majority of patients initiating BGF experienced real-life medication success reflecting the absence of severe cardiopulmonary events. These benefits were apparent after 90-days of treatment and sustained over 180-days.
Keywords: death; exacerbation; heart failure; myocardial infarction; pneumonia.
© 2024 Müllerová et al.
Conflict of interest statement
Hana Müllerová, Stefan Franzén, Johann Castaneda, and Jonathan Marshall are employees of, and own stock in, AstraZeneca. Jeffrey Shi Kai Chan, Heath Heatley, Victoria Carter, John Townend, and Derek Skinner are employees of Observational and Pragmatic Research Institute. David Price has advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; consultancy agreements with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; consultancy and lecture fees from Medscape, Inside Practice paid to Observational and Pragmatic Research Institute; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Chiesi, Viatris, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, and UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Viatris, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme, Teva Pharmaceuticals; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Novartis, Teva Pharmaceuticals; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation programme, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Triple Therapy with Budesonide/Glycopyrronium/Formoterol Fumarate Dihydrate versus Dual Therapies for Patients with COPD and Phenotypic Features of Asthma: A Pooled Post Hoc Analysis of KRONOS and ETHOS.Int J Chron Obstruct Pulmon Dis. 2024 Dec 12;19:2729-2737. doi: 10.2147/COPD.S478349. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 39691156 Free PMC article. Clinical Trial.
-
Characteristics of Patients with COPD Initiating Budesonide/Glycopyrronium/Formoterol or Other Triple Therapies in Japan: A Real-World Healthcare Claims Database Study (MITOS-AURA).Adv Ther. 2024 Dec;41(12):4518-4536. doi: 10.1007/s12325-024-02994-8. Epub 2024 Oct 16. Adv Ther. 2024. PMID: 39412626 Free PMC article.
-
Triple Therapy with Budesonide/Glycopyrrolate/Formoterol Fumarate Improves Inspiratory Capacity in Patients with Asthma-Chronic Obstructive Pulmonary Disease Overlap.Int J Chron Obstruct Pulmon Dis. 2020 Feb 5;15:269-277. doi: 10.2147/COPD.S231004. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 32103926 Free PMC article. Clinical Trial.
-
Triple therapy in COPD: new evidence with the extrafine fixed combination of beclomethasone dipropionate, formoterol fumarate, and glycopyrronium bromide.Int J Chron Obstruct Pulmon Dis. 2017 Oct 6;12:2917-2928. doi: 10.2147/COPD.S146822. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 29062229 Free PMC article. Review.
-
Spotlight on glycopyrronium/formoterol fumarate inhalation aerosol in the management of COPD: design, development, and place in therapy.Int J Chron Obstruct Pulmon Dis. 2017 Aug 3;12:2307-2312. doi: 10.2147/COPD.S89482. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28814858 Free PMC article. Review.
References
-
- Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2023. Available from: file:///C:/Users/Ruth%20Murray/Downloads/GOLD-2023-ver-1.3-17Feb2023_WMV.... Accessed March, 2024.
-
- Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. 2022;10(5):447–458. doi:10.1016/S2213-2600(21)00511-7 - DOI - PMC - PubMed
-
- World Health Organization. Chronic obstructive pulmonary disease. Available from: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pul...). Accessed March, 2024.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

