The efficacy and safety of EGFR-TKI in recurrent/metastatic nasopharyngeal carcinoma patients: A systematic review and meta-analysis
- PMID: 38803463
- PMCID: PMC11129551
- DOI: 10.1002/lio2.1279
The efficacy and safety of EGFR-TKI in recurrent/metastatic nasopharyngeal carcinoma patients: A systematic review and meta-analysis
Abstract
Objectives: EGFR-tyrosine kinase inhibitor (TKI) is used to treat recurrent and metastatic nasopharyngeal carcinoma (rmNPC). This meta-analysis aims to study the efficacy and safety of EGFR-TKI in treating patients with rmNPC.
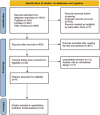
Methods: We conducted a systematic search of PubMed, Embase, and Web of Science up to November 2023, and included literature that met the criteria. We extracted objective response rate (ORR), disease control rate (DCR), median progression-free survival (mPFS), median overall survival (mOS), and adverse reaction-related events and performed meta-analysis using Stata 14.0.
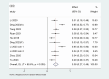
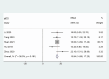
Results: A total of nine articles were included. The summary results showed that the ORR for patients treated with EGFR-TKI for rmNPC was 38% (95% CI = 27%-49%), the DCR was 71% (95% CI = 61%-80%), the mPFS was 6.29 months (95% CI = 5.22-7.35), and the mOS was 15.94 months (95% CI = 14.68-17.20). The most common grade 3-4 adverse reaction events in these patients were mucositis, nasopharyngeal necrosis, and oral ulceration. We found an incidence rate of 49% (95% CI = 38%-61%) for grade 3-4 adverse events (AEs). The anti-PD1 combined with TKI treatment method is more effective than the EGFR-TKI alone for treating rmNPC.
Conclusion: The study shows that EGFR-TKI has good efficacy in treating rmNPC but does not translate into survival benefits and owns a high incidence of grade 3-4 AEs. More RCT trials are needed in the future to verify the efficacy of anti-PD1 combined with TKI treatment method.
Keywords: EGFR‐TKI; anti‐PD1; immunotherapy; meta‐analysis; nasopharyngeal carcinoma; systematic review.
© 2024 The Author(s). Laryngoscope Investigative Otolaryngology published by Wiley Periodicals LLC on behalf of The Triological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
The efficacy and safety of VEGF/VEGFR inhibitors in patients with recurrent or metastatic nasopharyngeal carcinoma: A meta-analysis.Oral Oncol. 2022 Dec;135:106231. doi: 10.1016/j.oraloncology.2022.106231. Epub 2022 Oct 31. Oral Oncol. 2022. PMID: 36327674 Review.
-
The efficacy and safety of apatinib in patients with recurrent or metastatic nasopharyngeal carcinoma: a systematic review and meta-analysis.Transl Cancer Res. 2022 Jun;11(6):1770-1780. doi: 10.21037/tcr-22-1467. Transl Cancer Res. 2022. PMID: 35836539 Free PMC article.
-
Efficacy and Safety of Anti-PD1/PDL1 in Advanced Biliary Tract Cancer: A Systematic Review and Meta-Analysis.Front Immunol. 2022 Mar 2;13:801909. doi: 10.3389/fimmu.2022.801909. eCollection 2022. Front Immunol. 2022. PMID: 35309350 Free PMC article.
-
Feasibility and safety of EGFR-TKI neoadjuvant therapy for EGFR-mutated NSCLC: A meta-analysis.Eur J Clin Pharmacol. 2024 Apr;80(4):505-517. doi: 10.1007/s00228-024-03620-w. Epub 2024 Feb 1. Eur J Clin Pharmacol. 2024. PMID: 38300281 Review.
-
Efficacy of immunotherapy in patients with oncogene-driven non-small-cell lung cancer: a systematic review and meta-analysis.Ther Adv Med Oncol. 2024 Feb 27;16:17588359231225036. doi: 10.1177/17588359231225036. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 38420602 Free PMC article. Review.
References
-
- Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64‐80. - PubMed
-
- Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open‐label, phase 3 trial. Lancet. 2016;388(10054):1883‐1892. - PubMed
-
- Li WF, Chen NY, Zhang N, et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: long‐term results of phase 3 randomized controlled trial. Int J Cancer. 2019;145:295‐305. - PubMed
-
- Chen XS, Liang RB, Zhu XD. Anti‐EGFR therapies in nasopharyngeal carcinoma. Biomed Pharmacother. 2020;131:110649. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
