The heparin-binding domain of VEGF165 directly binds to integrin αvβ3 and VEGFR2/KDR D1: a potential mechanism of negative regulation of VEGF165 signaling by αvβ3
- PMID: 38803393
- PMCID: PMC11128890
- DOI: 10.3389/fcell.2024.1347616
The heparin-binding domain of VEGF165 directly binds to integrin αvβ3 and VEGFR2/KDR D1: a potential mechanism of negative regulation of VEGF165 signaling by αvβ3
Abstract
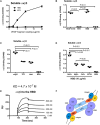
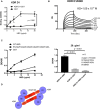
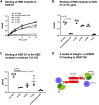
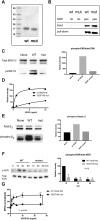
VEGF-A is a key cytokine in tumor angiogenesis and a major therapeutic target for cancer. VEGF165 is the predominant isoform of VEGF-A, and it is the most potent angiogenesis stimulant. VEGFR2/KDR domains 2 and 3 (D2D3) bind to the N-terminal domain (NTD, residues 1-110) of VEGF165. Since removal of the heparin-binding domain (HBD, residues 111-165) markedly reduced the mitogenic activity of the growth factor, it has been proposed that the HBD plays a critical role in the mitogenicity of VEGF165. Here, we report that αvβ3 specifically bound to the isolated VEGF165 HBD but not to VEGF165 NTD. Based on docking simulation and mutagenesis, we identified several critical amino acid residues within the VEGF165 HBD required for αvβ3 binding, i.e., Arg123, Arg124, Lys125, Lys140, Arg145, and Arg149. We discovered that VEGF165 HBD binds to the KDR domain 1 (D1) and identified that Arg123 and Arg124 are critical for KDR D1 binding by mutagenesis, indicating that the KDR D1-binding and αvβ3-binding sites overlap in the HBD. Full-length VEGF165 mutant (R123A/R124A/K125A/K140A/R145A/R149A) defective in αvβ3 and KDR D1 binding failed to induce ERK1/2 phosphorylation, integrin β3 phosphorylation, and KDR phosphorylation and did not support proliferation of endothelial cells, although the mutation did not affect the KDR D2D3 interaction with VEGF165. Since β3-knockout mice are known to show enhanced VEGF165 signaling, we propose that the binding of KDR D1 to the VEGF165 HBD and KDR D2D3 binding to the VEGF165 NTD are critically involved in the potent mitogenicity of VEGF165. We propose that binding competition between KDR and αvβ3 to the VEGF165 HBD endows integrin αvβ3 with regulatory properties to act as a negative regulator of VEGF165 signaling.
Keywords: VEGF receptor; VEGF165; angiogenesis; integrin; mutagenesis.
Copyright © 2024 Takada, Yu, Ye, Wu, Felding, Fujita and Takada.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Update of
-
The heparin-binding domain of VEGF165 directly binds to integrin αvβ3 and plays a critical role in signaling.bioRxiv [Preprint]. 2023 Nov 14:2023.11.14.567104. doi: 10.1101/2023.11.14.567104. bioRxiv. 2023. Update in: Front Cell Dev Biol. 2024 May 09;12:1347616. doi: 10.3389/fcell.2024.1347616 PMID: 38014319 Free PMC article. Updated. Preprint.
Similar articles
-
The heparin-binding domain of VEGF165 directly binds to integrin αvβ3 and plays a critical role in signaling.bioRxiv [Preprint]. 2023 Nov 14:2023.11.14.567104. doi: 10.1101/2023.11.14.567104. bioRxiv. 2023. Update in: Front Cell Dev Biol. 2024 May 09;12:1347616. doi: 10.3389/fcell.2024.1347616 PMID: 38014319 Free PMC article. Updated. Preprint.
-
Characterization of a soluble vascular endothelial growth factor receptor-immunoglobulin chimera.Growth Factors. 1997;14(4):243-56. doi: 10.3109/08977199709021523. Growth Factors. 1997. PMID: 9386989
-
Inhibition of vascular endothelial growth factor (VEGF)-induced endothelial cell proliferation by a peptide corresponding to the exon 7-encoded domain of VEGF165.J Biol Chem. 1997 Dec 12;272(50):31582-8. doi: 10.1074/jbc.272.50.31582. J Biol Chem. 1997. PMID: 9395496
-
Platelet factor-4 inhibits the mitogenic activity of VEGF121 and VEGF165 using several concurrent mechanisms.J Biol Chem. 1995 Jun 23;270(25):15059-65. doi: 10.1074/jbc.270.25.15059. J Biol Chem. 1995. PMID: 7797488
-
131I-Labeled arginine-arginine-leucine (RRL)-containing cyclic peptide (YCGGRRLGGC) for imaging prostate carcinoma.2010 Jan 6 [updated 2010 Feb 16]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2010 Jan 6 [updated 2010 Feb 16]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641373 Free Books & Documents. Review.
Cited by
-
FGF1 Suppresses Allosteric Activation of β3 Integrins by FGF2: A Potential Mechanism of Anti-Inflammatory and Anti-Thrombotic Action of FGF1.Biomolecules. 2024 Jul 23;14(8):888. doi: 10.3390/biom14080888. Biomolecules. 2024. PMID: 39199276 Free PMC article.
References
-
- Artoni A., Li J., Mitchell B., Ruan J., Takagi J., Springer T. A., et al. (2004). Integrin beta3 regions controlling binding of murine mAb 7E3: implications for the mechanism of integrin alphaIIbbeta3 activation. Proc. Natl. Acad. Sci. U. S. A. 101 (36), 13114–13120. 10.1073/pnas.0404201101 - DOI - PMC - PubMed
-
- Cedano Prieto D. M., Cheng Y., Chang C. C., Yu J., Takada Y. K., Takada Y. (2017). Direct integrin binding to insulin-like growth factor-2 through the C-domain is required for insulin-like growth factor receptor type 1 (IGF1R) signaling. PLoS One 12 (9), e0184285. 10.1371/journal.pone.0184285 - DOI - PMC - PubMed
-
- de Laat S. W., Boonstra J., Defize L. H., Kruijer W., van der Saag P. T., Tertoolen L. G., et al. (1999). Growth factor signalling. Int. J. Dev. Biol. 43 (7), 681–691. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

