Neither injury induced macrophages within the nerve, nor the environment created by Wallerian degeneration is necessary for enhanced in vivo axon regeneration after peripheral nerve injury
- PMID: 38802868
- PMCID: PMC11131297
- DOI: 10.1186/s12974-024-03132-5
Neither injury induced macrophages within the nerve, nor the environment created by Wallerian degeneration is necessary for enhanced in vivo axon regeneration after peripheral nerve injury
Abstract
Background: Since the 1990s, evidence has accumulated that macrophages promote peripheral nerve regeneration and are required for enhancing regeneration in the conditioning lesion (CL) response. After a sciatic nerve injury, macrophages accumulate in the injury site, the nerve distal to that site, and the axotomized dorsal root ganglia (DRGs). In the peripheral nervous system, as in other tissues, the macrophage response is derived from both resident macrophages and recruited monocyte-derived macrophages (MDMs). Unresolved questions are: at which sites do macrophages enhance nerve regeneration, and is a particular population needed.
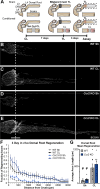
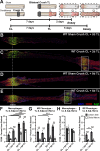
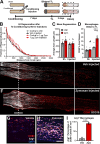
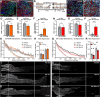
Methods: Ccr2 knock-out (KO) and Ccr2gfp/gfp knock-in/KO mice were used to prevent MDM recruitment. Using these strains in a sciatic CL paradigm, we examined the necessity of MDMs and residents for CL-enhanced regeneration in vivo and characterized injury-induced nerve inflammation. CL paradigm variants, including the addition of pharmacological macrophage depletion methods, tested the role of various macrophage populations in initiating or sustaining the CL response. In vivo regeneration, measured from bilateral proximal test lesions (TLs) after 2 d, and macrophages were quantified by immunofluorescent staining.
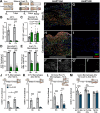
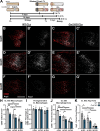
Results: Peripheral CL-enhanced regeneration was equivalent between crush and transection CLs and was sustained for 28 days in both Ccr2 KO and WT mice despite MDM depletion. Similarly, the central CL response measured in dorsal roots was unchanged in Ccr2 KO mice. Macrophages at both the TL and CL, but not between them, stained for the pro-regenerative marker, arginase 1. TL macrophages were primarily CCR2-dependent MDMs and nearly absent in Ccr2 KO and Ccr2gfp/gfp KO mice. However, there were only slightly fewer Arg1+ macrophages in CCR2 null CLs than controls due to resident macrophage compensation. Zymosan injection into an intact WT sciatic nerve recruited Arg1+ macrophages but did not enhance regeneration. Finally, clodronate injection into Ccr2gfp KO CLs dramatically reduced CL macrophages. Combined with the Ccr2gfp KO background, depleting MDMs and TL macrophages, and a transection CL, physically removing the distal nerve environment, nearly all macrophages in the nerve were removed, yet CL-enhanced regeneration was not impaired.
Conclusions: Macrophages in the sciatic nerve are neither necessary nor sufficient to produce a CL response.
Keywords: Arginase 1; Axotomy; CCR2; Clodronate; Conditioning lesion; Dorsal root; Macrophages; Neuroimmune; Regeneration; Zymosan.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
The primary macrophage chemokine, CCL2, is not necessary after a peripheral nerve injury for macrophage recruitment and activation or for conditioning lesion enhanced peripheral regeneration.J Neuroinflammation. 2022 Jul 12;19(1):179. doi: 10.1186/s12974-022-02497-9. J Neuroinflammation. 2022. PMID: 35820932 Free PMC article.
-
Analysis of the immune response to sciatic nerve injury identifies efferocytosis as a key mechanism of nerve debridement.Elife. 2020 Dec 2;9:e60223. doi: 10.7554/eLife.60223. Elife. 2020. PMID: 33263277 Free PMC article.
-
Neutrophils Are Critical for Myelin Removal in a Peripheral Nerve Injury Model of Wallerian Degeneration.J Neurosci. 2017 Oct 25;37(43):10258-10277. doi: 10.1523/JNEUROSCI.2085-17.2017. Epub 2017 Sep 14. J Neurosci. 2017. PMID: 28912156 Free PMC article.
-
Macrophage biology in the peripheral nervous system after injury.Prog Neurobiol. 2019 Feb;173:102-121. doi: 10.1016/j.pneurobio.2018.12.001. Epub 2018 Dec 21. Prog Neurobiol. 2019. PMID: 30579784 Free PMC article. Review.
-
Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury.Acta Neuropathol. 2015 Nov;130(5):605-18. doi: 10.1007/s00401-015-1482-4. Epub 2015 Sep 29. Acta Neuropathol. 2015. PMID: 26419777 Review.
Cited by
-
Immune drivers of pain resolution and protection.Nat Immunol. 2024 Dec;25(12):2200-2208. doi: 10.1038/s41590-024-02002-9. Epub 2024 Nov 11. Nat Immunol. 2024. PMID: 39528810 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

