Nanoparticle-Mediated Synergistic Chemoimmunotherapy for Cancer Treatment
- PMID: 38799699
- PMCID: PMC11127654
- DOI: 10.2147/IJN.S455213
Nanoparticle-Mediated Synergistic Chemoimmunotherapy for Cancer Treatment
Abstract
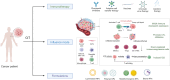
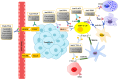
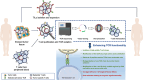
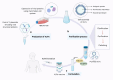
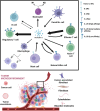
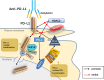
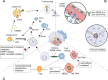
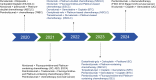
Until now, there has been a lack of effective strategies for cancer treatment. Immunotherapy has high potential in treating several cancers but its efficacy is limited as a monotherapy. Chemoimmunotherapy (CIT) holds promise to be widely used in cancer treatment. Therefore, identifying their involvement and potential synergy in CIT approaches is decisive. Nano-based drug delivery systems (NDDSs) are ideal delivery systems because they can simultaneously target immune cells and cancer cells, promoting drug accumulation, and reducing the toxicity of the drug. In this review, we first introduce five current immunotherapies, including immune checkpoint blocking (ICB), adoptive cell transfer therapy (ACT), cancer vaccines, oncolytic virus therapy (OVT) and cytokine therapy. Subsequently, the immunomodulatory effects of chemotherapy by inducing immunogenic cell death (ICD), promoting tumor killer cell infiltration, down-regulating immunosuppressive cells, and inhibiting immune checkpoints have been described. Finally, the NDDSs-mediated collaborative drug delivery systems have been introduced in detail, and the development of NDDSs-mediated CIT nanoparticles has been prospected.
Keywords: NDDSs; chemoimmunotherapy; chemotherapy; immunotherapy.
© 2024 Lang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Combining Oncolytic Viruses With Cancer Immunotherapy: Establishing a New Generation of Cancer Treatment.Front Immunol. 2020 Apr 28;11:683. doi: 10.3389/fimmu.2020.00683. eCollection 2020. Front Immunol. 2020. PMID: 32411132 Free PMC article. Review.
-
Overcoming T Cell Exhaustion in Tumor Microenvironment via Immune Checkpoint Modulation with Nano-Delivery Systems for Enhanced Immunotherapy.Small Methods. 2024 Aug;8(8):e2301326. doi: 10.1002/smtd.202301326. Epub 2023 Dec 1. Small Methods. 2024. PMID: 38040834 Review.
-
Combination strategies to maximize the benefits of cancer immunotherapy.J Hematol Oncol. 2021 Sep 27;14(1):156. doi: 10.1186/s13045-021-01164-5. J Hematol Oncol. 2021. PMID: 34579759 Free PMC article. Review.
-
Advances in the Study of Antitumour Immunotherapy for Newcastle Disease Virus.Int J Med Sci. 2021 Mar 30;18(11):2294-2302. doi: 10.7150/ijms.59185. eCollection 2021. Int J Med Sci. 2021. PMID: 33967605 Free PMC article. Review.
-
Nanoparticle-mediated synergistic chemoimmunotherapy for tailoring cancer therapy: recent advances and perspectives.J Nanobiotechnology. 2021 Apr 17;19(1):110. doi: 10.1186/s12951-021-00861-0. J Nanobiotechnology. 2021. PMID: 33865432 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

