This is a preprint.
Control of Inflammatory Response by Tissue Microenvironment
- PMID: 38798655
- PMCID: PMC11118380
- DOI: 10.1101/2024.05.10.592432
Control of Inflammatory Response by Tissue Microenvironment
Abstract
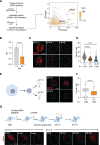
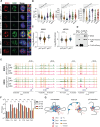
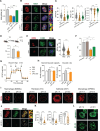
Inflammation is an essential defense response but operates at the cost of normal functions. Whether and how the negative impact of inflammation is monitored remains largely unknown. Acidification of the tissue microenvironment is associated with inflammation. Here we investigated whether macrophages sense tissue acidification to adjust inflammatory responses. We found that acidic pH restructured the inflammatory response of macrophages in a gene-specific manner. We identified mammalian BRD4 as a novel intracellular pH sensor. Acidic pH disrupts the transcription condensates containing BRD4 and MED1, via histidine-enriched intrinsically disordered regions. Crucially, decrease in macrophage intracellular pH is necessary and sufficient to regulate transcriptional condensates in vitro and in vivo, acting as negative feedback to regulate the inflammatory response. Collectively, these findings uncovered a pH-dependent switch in transcriptional condensates that enables environmental sensing to directly control inflammation, with a broader implication for calibrating the magnitude and quality of inflammation by the inflammatory cost.
Conflict of interest statement
Declaration of interests J.C.K. consults and holds equity in Corner Therapeutics and Larkspur Biosciences. None of these relationships impacted this study. The authors declare no competing interests.
Figures




Similar articles
-
Molecular features driving condensate formation and gene expression by the BRD4-NUT fusion oncoprotein are overlapping but distinct.Sci Rep. 2023 Jul 24;13(1):11907. doi: 10.1038/s41598-023-39102-9. Sci Rep. 2023. PMID: 37488172 Free PMC article.
-
Molecular features driving condensate formation and gene expression by the BRD4-NUT fusion oncoprotein are overlapping but distinct.bioRxiv [Preprint]. 2023 May 11:2023.05.11.540414. doi: 10.1101/2023.05.11.540414. bioRxiv. 2023. Update in: Sci Rep. 2023 Jul 24;13(1):11907. doi: 10.1038/s41598-023-39102-9. PMID: 37214845 Free PMC article. Updated. Preprint.
-
Phase separation of PML/RARα and BRD4 coassembled microspeckles governs transcriptional dysregulation in acute promyelocytic leukemia.Proc Natl Acad Sci U S A. 2024 Aug 20;121(34):e2406519121. doi: 10.1073/pnas.2406519121. Epub 2024 Aug 13. Proc Natl Acad Sci U S A. 2024. PMID: 39136995 Free PMC article.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Regulation of inflammation by extracellular acidification and proton-sensing GPCRs.Cell Signal. 2013 Nov;25(11):2263-71. doi: 10.1016/j.cellsig.2013.07.022. Epub 2013 Jul 31. Cell Signal. 2013. PMID: 23917207 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
