This is a preprint.
Stress-induced dysfunction of neurovascular astrocytes contributes to sex-specific behavioral deficits
- PMID: 38798398
- PMCID: PMC11118421
- DOI: 10.1101/2024.05.14.594147
Stress-induced dysfunction of neurovascular astrocytes contributes to sex-specific behavioral deficits
Abstract
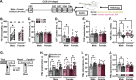
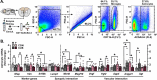
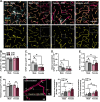
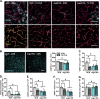
Astrocytes form an integral component of the neurovascular unit, ensheathing brain blood vessels with projections high in aquaporin-4 (AQP4) expression. These AQP4-rich projections facilitate interaction between the vascular endothelium, astrocytes, and neurons, and help stabilize vascular morphology. Studies using preclinical models of psychological stress and post-mortem tissue from patients with major depressive disorder (MDD) have reported reductions in AQP4, loss of astrocytic structures, and vascular impairment in the prefrontal cortex (PFC). Though compelling, the role of AQP4 in mediating stress-induced alterations in blood vessel function and behavior remains unclear. Here, we address this, alongside potential sex differences in chronic unpredictable stress (CUS) effects on astrocyte phenotype, blood-brain barrier integrity, and behavior. CUS led to pronounced shifts in stress-coping behavior and working memory deficits in male -but not female- mice. Following behavioral testing, astrocytes from the frontal cortex were isolated for gene expression analyses. We found that CUS increased various transcripts associated with blood vessel maintenance in astrocytes from males, but either had no effect on- or decreased- these genes in females. Furthermore, CUS caused a reduction in vascular-localized AQP4 and elevated extravasation of a small molecule fluorescent reporter (Dextran) in the PFC in males but not females. Studies showed that knockdown of AQP4 in the PFC in males is sufficient to disrupt astrocyte phenotype and increase behavioral susceptibility to a sub-chronic stressor. Collectively, these findings provide initial evidence that sex-specific alterations in astrocyte phenotype and neurovascular integrity in the PFC contribute to behavioral and cognitive consequences following chronic stress.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures

Similar articles
-
Synaptic and behavioral effects of chronic stress are linked to dynamic and sex-specific changes in microglia function and astrocyte dystrophy.Neurobiol Stress. 2021 Mar 4;14:100312. doi: 10.1016/j.ynstr.2021.100312. eCollection 2021 May. Neurobiol Stress. 2021. PMID: 33748354 Free PMC article.
-
Stress-Induced Neuronal Colony Stimulating Factor 1 Provokes Microglia-Mediated Neuronal Remodeling and Depressive-like Behavior.Biol Psychiatry. 2018 Jan 1;83(1):38-49. doi: 10.1016/j.biopsych.2017.05.026. Epub 2017 Jun 12. Biol Psychiatry. 2018. PMID: 28697890 Free PMC article.
-
Glucocorticoid receptor antagonism prevents microglia-mediated neuronal remodeling and behavioral despair following chronic unpredictable stress.Brain Behav Immun. 2019 Oct;81:329-340. doi: 10.1016/j.bbi.2019.06.030. Epub 2019 Jun 27. Brain Behav Immun. 2019. PMID: 31255679
-
AQP4 gene deletion in mice does not alter blood-brain barrier integrity or brain morphology.Neuroscience. 2009 Jul 7;161(3):764-72. doi: 10.1016/j.neuroscience.2009.03.069. Epub 2009 Apr 5. Neuroscience. 2009. PMID: 19345723 Review.
-
Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue.Curr Drug Targets. 2013 Oct;14(11):1225-36. doi: 10.2174/13894501113149990156. Curr Drug Targets. 2013. PMID: 23469922 Free PMC article. Review.
References
-
- Kobayashi H, Minami S, Itoh S, Shiraishi S, Yokoo H, Yanagita T, et al. Aquaporin subtypes in rat cerebral microvessels. Neuroscience Letters. 2001;297:163–166. - PubMed
-
- Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in Brain: Distribution, Physiology, and Pathophysiology. J Cereb Blood Flow Metab. 2002;22:367–378. - PubMed
-
- Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2006;1758:1085–1093. - PubMed
-
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
