Very Broadly Effective Hemagglutinin-Directed Influenza Vaccines with Anti-Herpetic Activity
- PMID: 38793788
- PMCID: PMC11125745
- DOI: 10.3390/vaccines12050537
Very Broadly Effective Hemagglutinin-Directed Influenza Vaccines with Anti-Herpetic Activity
Abstract
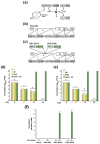
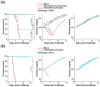
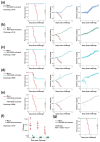
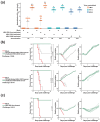
A universal vaccine that generally prevents influenza virus infection and/or illness remains elusive. We have been exploring a novel approach to vaccination involving replication-competent controlled herpesviruses (RCCVs) that can be deliberately activated to replicate efficiently but only transiently in an administration site in the skin of a subject. The RCCVs are derived from a virulent wild-type herpesvirus strain that has been engineered to contain a heat shock promoter-based gene switch that controls the expression of, typically, two replication-essential viral genes. Additional safety against inadvertent replication is provided by an appropriate secondary mechanism. Our first-generation RCCVs can be activated at the administration site by a mild local heat treatment in the presence of an antiprogestin. Here, we report that epidermal vaccination with such RCCVs expressing a hemagglutinin or neuraminidase of an H1N1 influenza virus strain protected mice against lethal challenges by H1N1 virus strains representing 75 years of evolution. Moreover, immunization with an RCCV expressing a subtype H1 hemagglutinin afforded full protection against a lethal challenge by an H3N2 influenza strain, and an RCCV expressing a subtype H3 hemagglutinin protected against a lethal challenge by an H1N1 strain. Vaccinated animals continued to gain weight normally after the challenge. Protective effects were even observed in a lethal influenza B virus challenge. The RCCV-based vaccines induced robust titers of in-group, cross-group and even cross-type neutralizing antibodies. Passive immunization suggested that observed vaccine effects were at least partially antibody-mediated. In summary, RCCVs expressing a hemagglutinin induce robust and very broad cross-protective immunity against influenza.
Keywords: broad protection; conditionally replicating; influenza; regulated; replication-competent; universal flu vaccine; vaccine; vectored vaccine.
Conflict of interest statement
Richard Voellmy is the founder of HSF Pharmaceuticals SA. The company had no role in data collection and interpretation. HSF Pharmaceuticals SA has property interests in RCCV technology. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
A Broad Influenza Vaccine Based on a Heat-Activated, Tissue-Restricted Replication-Competent Herpesvirus.Vaccines (Basel). 2024 Jun 23;12(7):703. doi: 10.3390/vaccines12070703. Vaccines (Basel). 2024. PMID: 39066341 Free PMC article.
-
Herpes Simplex Viruses Whose Replication Can Be Deliberately Controlled as Candidate Vaccines.Vaccines (Basel). 2020 May 18;8(2):230. doi: 10.3390/vaccines8020230. Vaccines (Basel). 2020. PMID: 32443425 Free PMC article. Review.
-
A pandemic influenza H1N1 live vaccine based on modified vaccinia Ankara is highly immunogenic and protects mice in active and passive immunizations.PLoS One. 2010 Aug 16;5(8):e12217. doi: 10.1371/journal.pone.0012217. PLoS One. 2010. PMID: 20808939 Free PMC article.
-
Elicitation of Protective Antibodies against 20 Years of Future H3N2 Cocirculating Influenza Virus Variants in Ferrets Preimmune to Historical H3N2 Influenza Viruses.J Virol. 2019 Jan 17;93(3):e00946-18. doi: 10.1128/JVI.00946-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429350 Free PMC article.
-
Bivalent H1 and H3 COBRA Recombinant Hemagglutinin Vaccines Elicit Seroprotective Antibodies against H1N1 and H3N2 Influenza Viruses from 2009 to 2019.J Virol. 2022 Apr 13;96(7):e0165221. doi: 10.1128/jvi.01652-21. Epub 2022 Mar 15. J Virol. 2022. PMID: 35289635 Free PMC article.
Cited by
-
A Broad Influenza Vaccine Based on a Heat-Activated, Tissue-Restricted Replication-Competent Herpesvirus.Vaccines (Basel). 2024 Jun 23;12(7):703. doi: 10.3390/vaccines12070703. Vaccines (Basel). 2024. PMID: 39066341 Free PMC article.
References
-
- Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S., Cohen C., Gran J.M., Schanzer D., Cowling B.J. Estimates of global seasonal influenza-associated respiratory mortality: A modeling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

