Integrin-Targeting Strategies for Adenovirus Gene Therapy
- PMID: 38793651
- PMCID: PMC11125847
- DOI: 10.3390/v16050770
Integrin-Targeting Strategies for Adenovirus Gene Therapy
Abstract
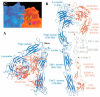
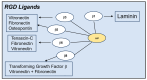
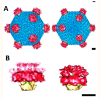
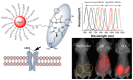
Numerous human adenovirus (AdV) types are endowed with arginine-glycine-aspartic acid (RGD) sequences that enable them to recognize vitronectin-binding (αv) integrins. These RGD-binding cell receptors mediate AdV entry into host cells, a crucial early step in virus infection. Integrin interactions with adenoviruses not only initiate receptor-mediated endocytosis but also facilitate AdV capsid disassembly, a prerequisite for membrane penetration by AdV protein VI. This review discusses fundamental aspects of AdV-host interactions mediated by integrins. Recent efforts to re-engineer AdV vectors and non-viral nanoparticles to target αv integrins for bioimaging and the eradication of cancer cells will also be discussed.
Keywords: adenovirus; bioimaging; extracellular matrix proteins; fiber; integrins; nanoparticles; penton base.
Conflict of interest statement
The author declares no conflicts of interest. The writing of the manuscript and the decision to publish prior data was solely that of the author.
Figures
Similar articles
-
Reduction of natural adenovirus tropism to mouse liver by fiber-shaft exchange in combination with both CAR- and alphav integrin-binding ablation.J Virol. 2003 Dec;77(24):13062-72. doi: 10.1128/jvi.77.24.13062-13072.2003. J Virol. 2003. PMID: 14645563 Free PMC article.
-
CAR- or alphav integrin-binding ablated adenovirus vectors, but not fiber-modified vectors containing RGD peptide, do not change the systemic gene transfer properties in mice.Gene Ther. 2002 Jun;9(12):769-76. doi: 10.1038/sj.gt.3301701. Gene Ther. 2002. PMID: 12040458
-
Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells.J Virol. 1996 Jul;70(7):4502-8. doi: 10.1128/JVI.70.7.4502-4508.1996. J Virol. 1996. PMID: 8676475 Free PMC article.
-
Adenovirus receptors and their implications in gene delivery.Virus Res. 2009 Aug;143(2):184-94. doi: 10.1016/j.virusres.2009.02.010. Epub 2009 Feb 26. Virus Res. 2009. PMID: 19647886 Free PMC article. Review.
-
Tropism and transduction of oncolytic adenovirus 5 vectors in cancer therapy: Focus on fiber chimerism and mosaicism, hexon and pIX.Virus Res. 2018 Sep 15;257:40-51. doi: 10.1016/j.virusres.2018.08.012. Epub 2018 Aug 17. Virus Res. 2018. PMID: 30125593 Review.
References
-
- Fu G., Wang W., Luo B. Overview: Structural biology of integrins. Methods Mol. Biol. 2012;757:81–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

