Cytokine Response of Natural Killer Cells to Hepatitis B Virus Infection Depends on Monocyte Co-Stimulation
- PMID: 38793623
- PMCID: PMC11125674
- DOI: 10.3390/v16050741
Cytokine Response of Natural Killer Cells to Hepatitis B Virus Infection Depends on Monocyte Co-Stimulation
Abstract
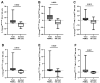
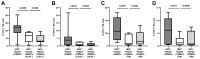
Hepatitis B virus (HBV) is a major driver of chronic hepatic inflammation, which regularly leads to liver cirrhosis or hepatocellular carcinoma. Immediate innate immune cell response is crucial for the rapid clearance of the infection. Here, natural killer (NK) cells play a pivotal role in direct cytotoxicity and the secretion of antiviral cytokines as well as regulatory function. The aim of this study was to further elucidate NK cell responses triggered by an HBV infection. Therefore, we optimized HBV in vitro models that reliably stimulate NK cells using hepatocyte-like HepG2 cells expressing the Na+-taurocholate co-transporting polypeptide (NTCP) and HepaRG cells. Immune cells were acquired from healthy platelet donors. Initially, HepG2-NTCP cells demonstrated higher viral replication compared to HepaRG cells. Co-cultures with immune cells revealed increased production of interferon-γ and tumor necrosis factor-α by NK cells, which was no longer evident in isolated NK cells. Likewise, the depletion of monocytes and spatial separation from target cells led to the absence of the antiviral cytokine production of NK cells. Eventually, the combined co-culture of isolated NK cells and monocytes led to a sufficient cytokine response of NK cells, which was also apparent when communication between the two immune cell subpopulations was restricted to soluble factors. In summary, our study demonstrates antiviral cytokine production by NK cells in response to HBV+ HepG2-NTCP cells, which is dependent on monocyte bystander activation.
Keywords: HBV; HepG2; HepaRG; NK cells; NTCP; bystander activation; hepatitis B virus; monocytes; natural killer cells.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Robust Human and Murine Hepatocyte Culture Models of Hepatitis B Virus Infection and Replication.J Virol. 2018 Nov 12;92(23):e01255-18. doi: 10.1128/JVI.01255-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30232184 Free PMC article.
-
NKG2D modulates aggravation of liver inflammation by activating NK cells in HBV infection.Sci Rep. 2017 Mar 7;7(1):88. doi: 10.1038/s41598-017-00221-9. Sci Rep. 2017. PMID: 28273905 Free PMC article.
-
Monocytes activate natural killer cells via inflammasome-induced interleukin 18 in response to hepatitis C virus replication.Gastroenterology. 2014 Jul;147(1):209-220.e3. doi: 10.1053/j.gastro.2014.03.046. Epub 2014 Mar 28. Gastroenterology. 2014. PMID: 24685721 Free PMC article.
-
HBV culture and infectious systems.Hepatol Int. 2016 Jul;10(4):559-66. doi: 10.1007/s12072-016-9712-y. Epub 2016 Mar 2. Hepatol Int. 2016. PMID: 26935052 Review.
-
Host-virus interactions in hepatitis B and hepatitis C infection.J Gastroenterol. 2016 May;51(5):409-20. doi: 10.1007/s00535-016-1183-3. Epub 2016 Feb 19. J Gastroenterol. 2016. PMID: 26894594 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

