Immunomodulatory Precision: A Narrative Review Exploring the Critical Role of Immune Checkpoint Inhibitors in Cancer Treatment
- PMID: 38791528
- PMCID: PMC11122264
- DOI: 10.3390/ijms25105490
Immunomodulatory Precision: A Narrative Review Exploring the Critical Role of Immune Checkpoint Inhibitors in Cancer Treatment
Abstract
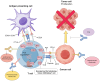
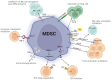
An immune checkpoint is a signaling pathway that regulates the recognition of antigens by T-cell receptors (TCRs) during an immune response. These checkpoints play a pivotal role in suppressing excessive immune responses and maintaining immune homeostasis against viral or microbial infections. There are several FDA-approved immune checkpoint inhibitors (ICIs), including ipilimumab, pembrolizumab, and avelumab. These ICIs target cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed death ligand 1 (PD-L1). Furthermore, ongoing efforts are focused on developing new ICIs with emerging potential. In comparison to conventional treatments, ICIs offer the advantages of reduced side effects and durable responses. There is growing interest in the potential of combining different ICIs with chemotherapy, radiation therapy, or targeted therapies. This article comprehensively reviews the classification, mechanism of action, application, and combination strategies of ICIs in various cancers and discusses their current limitations. Our objective is to contribute to the future development of more effective anticancer drugs targeting immune checkpoints.
Keywords: anti-cancer drugs; cancer treatment; combination immunotherapies; immune checkpoint inhibitors (ICIs); immune response.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Immune checkpoint inhibitors: breakthroughs in cancer treatment.Cancer Biol Med. 2024 May 24;21(6):451-72. doi: 10.20892/j.issn.2095-3941.2024.0055. Cancer Biol Med. 2024. PMID: 38801082 Free PMC article. Review.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
Immune Checkpoint Inhibitors for Patients Aged ≥ 75 Years with Advanced Cancer in First- and Second-Line Settings: A Meta-Analysis.Drugs Aging. 2020 Oct;37(10):747-754. doi: 10.1007/s40266-020-00788-5. Drugs Aging. 2020. PMID: 32681403
-
New insight in endocrine-related adverse events associated to immune checkpoint blockade.Best Pract Res Clin Endocrinol Metab. 2020 Jan;34(1):101370. doi: 10.1016/j.beem.2019.101370. Epub 2019 Dec 11. Best Pract Res Clin Endocrinol Metab. 2020. PMID: 31983543 Review.
-
Ileus in patients treated with immune checkpoint inhibitors: A retrospective, pharmacovigilance study using Food and Drug Administration Adverse Event Reporting System database.Pharmacoepidemiol Drug Saf. 2022 Nov;31(11):1199-1205. doi: 10.1002/pds.5493. Epub 2022 Jul 6. Pharmacoepidemiol Drug Saf. 2022. PMID: 35689298
Cited by
-
Advancements in Immunotherapeutic Treatments for Hepatocellular Carcinoma: Potential of Combination Therapies.Int J Mol Sci. 2024 Jun 21;25(13):6830. doi: 10.3390/ijms25136830. Int J Mol Sci. 2024. PMID: 38999940 Free PMC article. Review.
-
mRNA vaccines: a new era in vaccine development.Oncol Res. 2024 Sep 18;32(10):1543-1564. doi: 10.32604/or.2024.043987. eCollection 2024. Oncol Res. 2024. PMID: 39308511 Free PMC article. Review.
-
[Enhanced tumoricidal activity of PD-1 antibody-secreting c-Met CAR-T cells against pancreatic cancer cells].Nan Fang Yi Ke Da Xue Xue Bao. 2024 Oct 20;44(10):1976-1984. doi: 10.12122/j.issn.1673-4254.2024.10.16. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 39523098 Free PMC article. Chinese.
-
PI3K/AKT/mTOR and PD‑1/CTLA‑4/CD28 pathways as key targets of cancer immunotherapy (Review).Oncol Lett. 2024 Sep 26;28(6):567. doi: 10.3892/ol.2024.14700. eCollection 2024 Dec. Oncol Lett. 2024. PMID: 39390982 Free PMC article. Review.
-
Recent Treatment Strategies and Molecular Pathways in Resistance Mechanisms of Antiangiogenic Therapies in Glioblastoma.Cancers (Basel). 2024 Aug 27;16(17):2975. doi: 10.3390/cancers16172975. Cancers (Basel). 2024. PMID: 39272834 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

