Molecular Characterization and Inhibition of a Novel Stress-Induced Mitochondrial Protecting Role for Misfolded TrkAIII in Human SH-SY5Y Neuroblastoma Cells
- PMID: 38791513
- PMCID: PMC11122047
- DOI: 10.3390/ijms25105475
Molecular Characterization and Inhibition of a Novel Stress-Induced Mitochondrial Protecting Role for Misfolded TrkAIII in Human SH-SY5Y Neuroblastoma Cells
Abstract
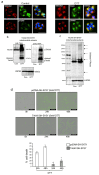
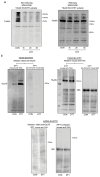
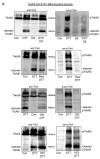
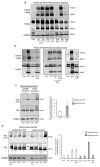
Pediatric neuroblastomas (NBs) are heterogeneous, aggressive, therapy-resistant embryonal tumors that originate from cells of neural crest origin committed to the sympathoadrenal progenitor cell lineage. Stress- and drug-resistance mechanisms drive post-therapeutic relapse and metastatic progression, the characterization and inhibition of which are major goals in improving therapeutic responses. Stress- and drug-resistance mechanisms in NBs include alternative TrkAIII splicing of the neurotrophin receptor tropomyosin-related kinase A (NTRK1/TrkA), which correlates with post-therapeutic relapse and advanced-stage metastatic disease. The TrkAIII receptor variant exerts oncogenic activity in NB models by mechanisms that include stress-induced mitochondrial importation and activation. In this study, we characterize novel targetable and non-targetable participants in this pro-survival mechanism in TrkAIII-expressing SH-SY5Y NB cells, using dithiothreitol (DTT) as an activator and a variety of inhibitors by regular and immunoprecipitation Western blotting of purified mitochondria and IncuCyte cytotoxicity assays. We report that stress-induced TrkAIII misfolding initiates this mechanism, resulting in Grp78, Ca2+-calmodulin, adenosine ribosylating factor (Arf) and Hsp90-regulated mitochondrial importation. TrkAIII imported into inner mitochondrial membranes is cleaved by Omi/high temperature requirement protein A2 (HtrA2) then activated by a mechanism dependent upon calmodulin kinase II (CaMKII), alpha serine/threonine kinase (Akt), mitochondrial Ca2+ uniporter and reactive oxygen species (ROS), involving inhibitory mitochondrial protein tyrosine phosphatase (PTPase) oxidation, resulting in phosphoinositide 3 kinase (PI3K) activation of mitochondrial Akt, which enhances stress resistance. This novel pro-survival function for misfolded TrkAIII mitigates the cytotoxicity of mitochondrial Ca2+ homeostasis disrupted during integrated stress responses, and is prevented by clinically approved Trk and Akt inhibitors and also by inhibitors of 78kDa glucose regulated protein (Grp78), heat shock protein 90 (Hsp90), Ca2+-calmodulin and PI3K. This identifies Grp78, Ca2+-calmodulin, Hsp90, PI3K and Akt as novel targetable participants in this mechanism, in addition to TrkAIII, the inhibition of which has the potential to enhance the stress-induced elimination of TrkAIII-expressing NB cells, with the potential to improve therapeutic outcomes in NBs that exhibit TrkAIII expression and activation.
Keywords: Akt; Ca2+-calmodulin; Grp78; PI3K; ROS; TrkAIII; adenosine ribosylating factor; integrated stress response; mitochondria; mitochondrial Ca2+ uniporter; stress resistance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









Similar articles
-
The effectiveness of school-based family asthma educational programs on the quality of life and number of asthma exacerbations of children aged five to 18 years diagnosed with asthma: a systematic review protocol.JBI Database System Rev Implement Rep. 2015 Oct;13(10):69-81. doi: 10.11124/jbisrir-2015-2335. JBI Database System Rev Implement Rep. 2015. PMID: 26571284
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Enabling Systemic Identification and Functionality Profiling for Cdc42 Homeostatic Modulators.bioRxiv [Preprint]. 2024 Jan 8:2024.01.05.574351. doi: 10.1101/2024.01.05.574351. bioRxiv. 2024. Update in: Commun Chem. 2024 Nov 19;7(1):271. doi: 10.1038/s42004-024-01352-7. PMID: 38260445 Free PMC article. Updated. Preprint.
-
Identification of a novel toxicophore in anti-cancer chemotherapeutics that targets mitochondrial respiratory complex I.Elife. 2020 May 20;9:e55845. doi: 10.7554/eLife.55845. Elife. 2020. PMID: 32432547 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

