The Role of microRNA in the Regulation of Cortisol Metabolism in the Adipose Tissue in the Course of Obesity
- PMID: 38791098
- PMCID: PMC11120731
- DOI: 10.3390/ijms25105058
The Role of microRNA in the Regulation of Cortisol Metabolism in the Adipose Tissue in the Course of Obesity
Abstract
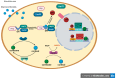
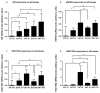
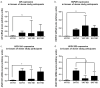
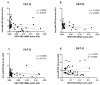
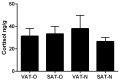
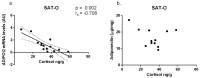
The similarity of the clinical picture of metabolic syndrome and hypercortisolemia supports the hypothesis that obesity may be associated with impaired expression of genes related to cortisol action and metabolism in adipose tissue. The expression of genes encoding the glucocorticoid receptor alpha (GR), cortisol metabolizing enzymes (HSD11B1, HSD11B2, H6PDH), and adipokines, as well as selected microRNAs, was measured by real-time PCR in adipose tissue from 75 patients with obesity, 19 patients following metabolic surgery, and 25 normal-weight subjects. Cortisol levels were analyzed by LC-MS/MS in 30 pairs of tissues. The mRNA levels of all genes studied were significantly (p < 0.05) decreased in the visceral adipose tissue (VAT) of patients with obesity and normalized by weight loss. In the subcutaneous adipose tissue (SAT), GR and HSD11B2 were affected by this phenomenon. Negative correlations were observed between the mRNA levels of the investigated genes and selected miRNAs (hsa-miR-142-3p, hsa-miR-561, and hsa-miR-579). However, the observed changes did not translate into differences in tissue cortisol concentrations, although levels of this hormone in the SAT of patients with obesity correlated negatively with mRNA levels for adiponectin. In conclusion, although the expression of genes related to cortisol action and metabolism in adipose tissue is altered in obesity and miRNAs may be involved in this process, these changes do not affect tissue cortisol concentrations.
Keywords: adipose tissue; cortisol metabolism; glucocorticoid receptor alpha; metabolic inflammation; microRNA; obesity.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
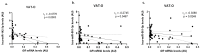


Similar articles
-
Changes in adipose glucocorticoid metabolism before and after bariatric surgery assessed by direct hormone measurements.Obesity (Silver Spring). 2013 Dec;21(12):2495-503. doi: 10.1002/oby.20449. Epub 2013 Jun 13. Obesity (Silver Spring). 2013. PMID: 23512832
-
The expression of 11β-HSDs, GR, and H6PDH in subcutaneous adipose tissue from polycystic ovary syndrome subjects.Horm Metab Res. 2013 Oct;45(11):802-7. doi: 10.1055/s-0033-1345186. Epub 2013 Aug 26. Horm Metab Res. 2013. PMID: 23979790
-
Expression of genes related to glucocorticoid action in human subcutaneous and omental adipose tissue.J Steroid Biochem Mol Biol. 2010 Oct;122(1-3):28-34. doi: 10.1016/j.jsbmb.2010.02.024. Epub 2010 Mar 3. J Steroid Biochem Mol Biol. 2010. PMID: 20206259
-
Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity.Biochim Biophys Acta. 2014 Mar;1842(3):473-81. doi: 10.1016/j.bbadis.2013.05.029. Epub 2013 Jun 2. Biochim Biophys Acta. 2014. PMID: 23735216 Free PMC article. Review.
-
Tissue-specific dysregulation of cortisol regeneration by 11βHSD1 in obesity: has it promised too much?Diabetologia. 2014 Jun;57(6):1100-10. doi: 10.1007/s00125-014-3228-6. Epub 2014 Apr 8. Diabetologia. 2014. PMID: 24710966 Review.
References
-
- Pasquali R., Casanueva F., Haluzik M., van Hulsteijn L., Ledoux S., Monteiro M.P., Salvador J., Santini F., Toplak H., Dekkers O.M. European Society of Endocrinology Clinical Practice Guideline: Endocrine work-up in obesity. Eur. J. Endocrinol. 2020;182:G1–G32. doi: 10.1530/EJE-19-0893. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

