Microbe-Derived Antioxidants Protect IPEC-1 Cells from H2O2-Induced Oxidative Stress, Inflammation and Tight Junction Protein Disruption via Activating the Nrf2 Pathway to Inhibit the ROS/NLRP3/IL-1β Signaling Pathway
- PMID: 38790638
- PMCID: PMC11117695
- DOI: 10.3390/antiox13050533
Microbe-Derived Antioxidants Protect IPEC-1 Cells from H2O2-Induced Oxidative Stress, Inflammation and Tight Junction Protein Disruption via Activating the Nrf2 Pathway to Inhibit the ROS/NLRP3/IL-1β Signaling Pathway
Abstract
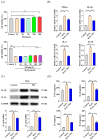
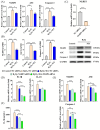
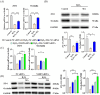
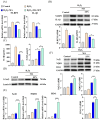
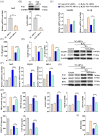
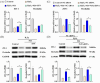
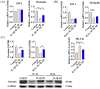
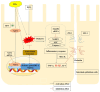
Oxidative stress can induce inflammation and tight junction disruption in enterocytes. The initiation of inflammation is thought to commence with the activation of the ROS/NLRP3/IL-1β signaling pathway, marking a crucial starting point in the process. In our previous studies, we found that microbe-derived antioxidants (MAs) showed significant potential in enhancing both antioxidant capabilities and anti-inflammatory effects. The main aim of this research was to investigate the ability of MAs to protect cells from oxidative stress caused by H2O2, to reduce inflammatory responses, and to maintain the integrity of tight junction proteins by modulating the ROS/NLRP3/IL-1β signaling pathway. IPEC-1 cells (1 × 104 cells/well) were initially exposed to 100 mg/L of MAs for 12 h, after which they were subjected to 1 mM H2O2 treatment for 1 h. We utilized small interfering RNA (siRNA) to inhibit the expression of NLRP3 and Nrf2. Inflammatory factors such as IL-1β and antioxidant enzyme activity levels were detected by ELISA. Oxidative stress marker ROS was examined by fluorescence analysis. The NLRP3/IL-1β signaling pathway, Nrf2/HO-1 signaling pathway and tight junction proteins (ZO-1 and Occludin) were detected by RT-qPCR or Western blotting. In our research, it was observed that MA treatment effectively suppressed the notable increase in H2O2-induced inflammatory markers (TNF-α, IL-1β, and IL-18), decreased ROS accumulation, mitigated the expression of NLRP3, ASC, and caspase-1, and promoted the expression of ZO-1 and Occludin. After silencing the NLRP3 gene with siRNA, the protective influence of MAs was observed to be linked with the NLRP3 inflammasome. Additional investigations demonstrated that the treatment with MAs triggered the activation of Nrf2, facilitating its translocation into the nucleus. This process resulted in a notable upregulation of Nrf2, NQO1, and HO-1 expression, along with the initiation of the Nrf2-HO-1 signaling pathway. Consequently, there was an enhancement in the activities of antioxidant enzymes like SOD, GSH-Px, and CAT, which effectively mitigated the accumulation of ROS, thereby ameliorating the oxidative stress state. The antioxidant effectiveness of MAs was additionally heightened in the presence of SFN, an activator of Nrf2. The antioxidant and anti-inflammatory functions of MAs and their role in regulating intestinal epithelial tight junction protein disruption were significantly affected after siRNA knockdown of the Nrf2 gene. These findings suggest that MAs have the potential to reduce H2O2-triggered oxidative stress, inflammation, and disruption of intestinal epithelial tight junction proteins in IPEC-1 cells. This reduction is achieved by blocking the ROS/NLRP3/IL-1β signaling pathway through the activation of the Nrf2 pathway.
Keywords: Nrf2/ROS/NLRP3/IL-1β; inflammatory response; microbe-derived antioxidants; oxidative stress; tight junction proteins.
Conflict of interest statement
All the authors declare that they have no conflicts of interest related to this study.
Figures
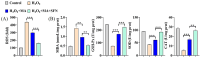
Similar articles
-
Microbe-Derived Antioxidants Reduce Lipopolysaccharide-Induced Inflammatory Responses by Activating the Nrf2 Pathway to Inhibit the ROS/NLRP3/IL-1β Signaling Pathway.Int J Mol Sci. 2022 Oct 18;23(20):12477. doi: 10.3390/ijms232012477. Int J Mol Sci. 2022. PMID: 36293333 Free PMC article.
-
Syringic acid suppresses Cutibacterium acnes-induced inflammation in human keratinocytes via regulating the NLRP3/caspase-1/IL-1β signaling axis by activating PPARγ/Nrf2-antioxidant pathway.Int Immunopharmacol. 2024 Sep 30;139:112708. doi: 10.1016/j.intimp.2024.112708. Epub 2024 Jul 20. Int Immunopharmacol. 2024. PMID: 39033661
-
Calcitriol inhibits ROS-NLRP3-IL-1β signaling axis via activation of Nrf2-antioxidant signaling in hyperosmotic stress stimulated human corneal epithelial cells.Redox Biol. 2019 Feb;21:101093. doi: 10.1016/j.redox.2018.101093. Epub 2018 Dec 26. Redox Biol. 2019. PMID: 30611121 Free PMC article.
-
Antioxidant Effects of Statins by Modulating Nrf2 and Nrf2/HO-1 Signaling in Different Diseases.J Clin Med. 2022 Feb 27;11(5):1313. doi: 10.3390/jcm11051313. J Clin Med. 2022. PMID: 35268403 Free PMC article. Review.
-
OxInflammatory Responses in the Wound Healing Process: A Systematic Review.Antioxidants (Basel). 2024 Jul 9;13(7):823. doi: 10.3390/antiox13070823. Antioxidants (Basel). 2024. PMID: 39061892 Free PMC article. Review.
Cited by
-
Regulatory Effects of Maternal Intake of Microbial-Derived Antioxidants on Colonization of Microbiota in Breastmilk and That of Intestinal Microbiota in Offspring.Animals (Basel). 2024 Sep 5;14(17):2582. doi: 10.3390/ani14172582. Animals (Basel). 2024. PMID: 39272367 Free PMC article.
References
-
- Luo J.F., Shen X.Y., Lio C.K., Dai Y., Cheng C.S., Liu J.X., Yao Y.D., Yu Y., Xie Y., Luo P., et al. Activation of Nrf2/HO-1 Pathway by Nardochinoid C Inhibits Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages. Front. Pharmacol. 2018;9:911. doi: 10.3389/fphar.2018.00911. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

