Extracellular Vesicles from Ecklonia cava and Phlorotannin Promote Rejuvenation in Aged Skin
- PMID: 38786614
- PMCID: PMC11123375
- DOI: 10.3390/md22050223
Extracellular Vesicles from Ecklonia cava and Phlorotannin Promote Rejuvenation in Aged Skin
Abstract
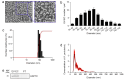
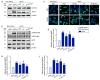
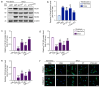
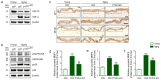
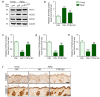
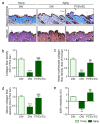
Plant-derived extracellular vesicles (EVs) elicit diverse biological effects, including promoting skin health. EVs isolated from Ecklonia cava (EV-EC) carry heat shock protein 70 (HSP70), which inhibits key regulators such as TNF-α, MAPKs, and NF-κB, consequently downregulating matrix metalloproteinases (MMPs). Aging exacerbates oxidative stress, upregulating MAPK and NF-κB signaling and worsening extracellular matrix degradation in the skin. E. cava-derived phlorotannin (PT) mitigates MAPK and NF-κB signaling. We evaluated the impact of EV-EC and PT on skin rejuvenation using an in vitro keratinocyte senescence model and an in vivo aged-mouse model. Western blotting confirmed the presence of HSP70 in EV-EC. Treatment with EV-EC and PT in senescent keratinocytes increased HSP70 expression and decreased the expression of TNF-α, MAPK, NF-κB, activator protein-1 (AP-1), and MMPs. Oxidative stress was also reduced. Sequential treatment with PT and EV-EC (PT/EV-EC) yielded more significant results compared to individual treatments. The administration of PT/EV-EC to the back skin of aged mice mirrored the in vitro findings, resulting in increased collagen fiber accumulation and improved elasticity in the aged skin. Therefore, PT/EV-EC holds promise in promoting skin rejuvenation by increasing HSP70 expression, decreasing the expression of MMPs, and reducing oxidative stress in aged skin.
Keywords: Ecklonia cava; extracellular vesicles from Ecklonia cava; phlorotannin; skin rejuvenation.
Conflict of interest statement
Kyunghee Byun has received research grants from SACCI Bio Co.
Figures

Similar articles
-
Co-Treatment with Phlorotannin and Extracellular Vesicles from Ecklonia cava Inhibits UV-Induced Melanogenesis.Antioxidants (Basel). 2024 Mar 28;13(4):408. doi: 10.3390/antiox13040408. Antioxidants (Basel). 2024. PMID: 38671856 Free PMC article.
-
Chitosan hydrogel-loaded MSC-derived extracellular vesicles promote skin rejuvenation by ameliorating the senescence of dermal fibroblasts.Stem Cell Res Ther. 2021 Mar 20;12(1):196. doi: 10.1186/s13287-021-02262-4. Stem Cell Res Ther. 2021. PMID: 33743829 Free PMC article.
-
Acer tataricum subsp. ginnala Inhibits Skin Photoaging via Regulating MAPK/AP-1, NF-κB, and TGFβ/Smad Signaling in UVB-Irradiated Human Dermal Fibroblasts.Molecules. 2021 Jan 27;26(3):662. doi: 10.3390/molecules26030662. Molecules. 2021. PMID: 33513930 Free PMC article.
-
NF-κB signaling in skin aging.Mech Ageing Dev. 2019 Dec;184:111160. doi: 10.1016/j.mad.2019.111160. Epub 2019 Oct 18. Mech Ageing Dev. 2019. PMID: 31634486 Review.
-
Protecting skin photoaging by NF-kappaB inhibitor.Curr Drug Metab. 2010 Jun 1;11(5):431-5. doi: 10.2174/138920010791526051. Curr Drug Metab. 2010. PMID: 20540695 Review.
References
-
- Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

