Reactive spinal glia convert 2-AG to prostaglandins to drive aberrant astroglial calcium signaling
- PMID: 38784707
- PMCID: PMC11112260
- DOI: 10.3389/fncel.2024.1382465
Reactive spinal glia convert 2-AG to prostaglandins to drive aberrant astroglial calcium signaling
Abstract
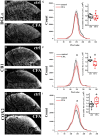
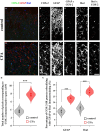
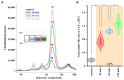
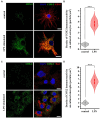
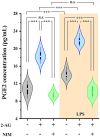
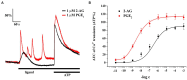
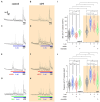
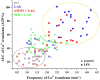
The endogenous cannabinoid 2-arachidonoylglycerol (2-AG) influences neurotransmission in the central nervous system mainly by activating type 1 cannabinoid receptor (CB1). Following its release, 2-AG is broken down by hydrolases to yield arachidonic acid, which may subsequently be metabolized by cyclooxygenase-2 (COX-2). COX-2 converts arachidonic acid and also 2-AG into prostanoids, well-known inflammatory and pro-nociceptive mediators. Here, using immunohistochemical and biochemical methods and pharmacological manipulations, we found that reactive spinal astrocytes and microglia increase the expression of COX-2 and the production of prostaglandin E2 when exposed to 2-AG. Both 2-AG and PGE2 evoke calcium transients in spinal astrocytes, but PGE2 showed 30% more efficacy and 55 times more potency than 2-AG. Unstimulated spinal dorsal horn astrocytes responded to 2-AG with calcium transients mainly through the activation of CB1. 2-AG induced exaggerated calcium transients in reactive astrocytes, but this increase in the frequency and area under the curve of calcium signals was only partially dependent on CB1. Instead, aberrant calcium transients were almost completely abolished by COX-2 inhibition. Our results suggest that both reactive spinal astrocytes and microglia perform an endocannabinoid-prostanoid switch to produce PGE2 at the expense of 2-AG. PGE2 in turn is responsible for the induction of aberrant astroglial calcium signals which, together with PGE2 production may play role in the development and maintenance of spinal neuroinflammation-associated disturbances such as central sensitization.
Keywords: 2-AG; CB1; COX-2; astrocyte; calcium signaling; cannabinoid; prostaglandin; reactive astrocyte.
Copyright © 2024 Dócs, Balázs, Papp, Szücs and Hegyi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
WWL70 attenuates PGE2 production derived from 2-arachidonoylglycerol in microglia by ABHD6-independent mechanism.J Neuroinflammation. 2017 Jan 10;14(1):7. doi: 10.1186/s12974-016-0783-4. J Neuroinflammation. 2017. PMID: 28086912 Free PMC article.
-
CB1 receptor activation induces intracellular Ca2+ mobilization and 2-arachidonoylglycerol release in rodent spinal cord astrocytes.Sci Rep. 2018 Jul 12;8(1):10562. doi: 10.1038/s41598-018-28763-6. Sci Rep. 2018. PMID: 30002493 Free PMC article.
-
WWL70 protects against chronic constriction injury-induced neuropathic pain in mice by cannabinoid receptor-independent mechanisms.J Neuroinflammation. 2018 Jan 8;15(1):9. doi: 10.1186/s12974-017-1045-9. J Neuroinflammation. 2018. PMID: 29310667 Free PMC article.
-
Spinal astrocytes as therapeutic targets for pathological pain.J Pharmacol Sci. 2010;114(4):347-53. doi: 10.1254/jphs.10r04cp. Epub 2010 Nov 11. J Pharmacol Sci. 2010. PMID: 21081837 Review.
-
Pathophysiological Roles of Cyclooxygenases and Prostaglandins in the Central Nervous System.Mol Neurobiol. 2016 Sep;53(7):4754-71. doi: 10.1007/s12035-015-9355-3. Epub 2015 Sep 2. Mol Neurobiol. 2016. PMID: 26328537 Review.
References
-
- Anrather J., Gallo E. F., Kawano T., Orio M., Abe T., Gooden C., et al. . (2011). Purinergic signaling induces cyclooxygenase-1-dependent prostanoid synthesis in microglia: roles in the outcome of excitotoxic brain injury. PLoS One 6:e25916. doi: 10.1371/journal.pone.0025916, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

