Variables affecting new drug prices in South Korea's pricing system
- PMID: 38783941
- PMCID: PMC11113548
- DOI: 10.3389/fphar.2024.1370915
Variables affecting new drug prices in South Korea's pricing system
Abstract
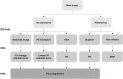
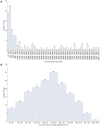
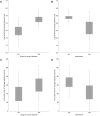
Objective: The price of pharmaceuticals is important from the economic and industrial perspectives but as well as patients' access to treatment. This study aimed to analyze the variables affecting the prices of new drugs in South Korea's pricing system. Methods: Data on 192 new drugs listed in South Korea from 2012 to 2022 were collected from the official website of the Health Insurance Review and Assessment Service. The independent variables included drugs for severe diseases, alternatives, number of patients, number of advanced 7 countries listed, budget impact, and listing period. The dependent variables included annual treatment cost and the price ratio to the advanced 7 country's average adjusted price. Descriptive statistics of variables, linear correlations between quantitative independent and dependent variables, and associations between independent and dependent variables were analyzed. Results: The mean annual treatment cost and price ratio to the advanced 7 country's average adjusted price were higher for drugs for severe diseases and those with no alternatives. Annual treatment cost and price ratio to the advanced 7 country's average adjusted price were negatively correlated with the number of patients and positively correlated with the number of advanced 7 countries listed. Annual treatment cost was affected by the variables drugs for severe diseases, alternatives, number of patients, number of advanced 7 countries listed, and budget impact. The price ratio to the advanced 7 country's average adjusted price was affected by drugs for severe diseases, alternatives, and the number of patients. Conclusion: This study revealed the effect of different variables on the prices of new drugs in South Korea, allowing for the development of a more effective assessment system to evaluate the prices of new drugs while ensuring profitability for pharmaceutical companies, sustainability of public insurance, and accessibility to drugs by patients.
Keywords: budget impact; cost-effectiveness; drug price; health technology assessment; patient accessibility; pricing.
Copyright © 2024 Lee, Cho, Lee, Kang and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Price increase negotiations to address drug shortages in South Korea's national health insurance.Front Pharmacol. 2023 Dec 4;14:1307462. doi: 10.3389/fphar.2023.1307462. eCollection 2023. Front Pharmacol. 2023. PMID: 38111380 Free PMC article.
-
Letter to the Editor: Rethinking The Cost Of Antipsychotic Treatment: The Average Cost Of The Drugs Used In Turkey In 2020.Turk Psikiyatri Derg. 2022 Summer;33(2):146-148. doi: 10.5080/u26315. Turk Psikiyatri Derg. 2022. PMID: 35730516 English, Turkish.
-
Pricing and Reimbursement Pathways of New Orphan Drugs in South Korea: A Longitudinal Comparison.Healthcare (Basel). 2021 Mar 8;9(3):296. doi: 10.3390/healthcare9030296. Healthcare (Basel). 2021. PMID: 33800373 Free PMC article.
-
Pharmaceutical policies: effects of reference pricing, other pricing, and purchasing policies.Cochrane Database Syst Rev. 2014 Oct 16;2014(10):CD005979. doi: 10.1002/14651858.CD005979.pub2. Cochrane Database Syst Rev. 2014. PMID: 25318966 Free PMC article. Review.
-
Impact of European pharmaceutical price regulation on generic price competition: a review.Pharmacoeconomics. 2010;28(8):649-63. doi: 10.2165/11535360-000000000-00000. Pharmacoeconomics. 2010. PMID: 20515079 Review.
References
-
- HIRA (2023a) Drug reimbursement and evaluation committee. Available at: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA030014040000 (Accessed July 3, 2023).
-
- HIRA (2023b) Regulations on evaluation criteria and procedures for eligibility of drugs on reimbursement. Available at: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA040055000000 (Accessed July 3, 2023).
-
- HIRA (2023c) Reimbursement history information. Available at: https://www.hira.or.kr/ra/medi/getHistoryList.do?pgmid=HIRAA030035020000 (Accessed July 3, 2023).
Grants and funding
LinkOut - more resources
Full Text Sources

