A closer look at mammalian antiviral condensates
- PMID: 38778761
- PMCID: PMC11234502
- DOI: 10.1042/BST20231296
A closer look at mammalian antiviral condensates
Abstract
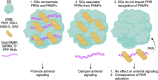
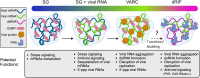
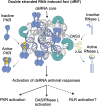
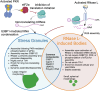
Several biomolecular condensates assemble in mammalian cells in response to viral infection. The most studied of these are stress granules (SGs), which have been proposed to promote antiviral innate immune signaling pathways, including the RLR-MAVS, the protein kinase R (PKR), and the OAS-RNase L pathways. However, recent studies have demonstrated that SGs either negatively regulate or do not impact antiviral signaling. Instead, the SG-nucleating protein, G3BP1, may function to perturb viral RNA biology by condensing viral RNA into viral-aggregated RNA condensates, thus explaining why viruses often antagonize G3BP1 or hijack its RNA condensing function. However, a recently identified condensate, termed double-stranded RNA-induced foci, promotes the activation of the PKR and OAS-RNase L antiviral pathways. In addition, SG-like condensates known as an RNase L-induced bodies (RLBs) have been observed during many viral infections, including SARS-CoV-2 and several flaviviruses. RLBs may function in promoting decay of cellular and viral RNA, as well as promoting ribosome-associated signaling pathways. Herein, we review these recent advances in the field of antiviral biomolecular condensates, and we provide perspective on the role of canonical SGs and G3BP1 during the antiviral response.
Keywords: G3BP1; PKR; RNA; RNase L; condensate; stress granules.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

Similar articles
-
RNase L Amplifies Interferon Signaling by Inducing Protein Kinase R-Mediated Antiviral Stress Granules.J Virol. 2020 Jun 16;94(13):e00205-20. doi: 10.1128/JVI.00205-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32295917 Free PMC article.
-
The stress granule protein G3BP1 recruits protein kinase R to promote multiple innate immune antiviral responses.J Virol. 2015 Mar;89(5):2575-89. doi: 10.1128/JVI.02791-14. Epub 2014 Dec 17. J Virol. 2015. PMID: 25520508 Free PMC article.
-
Stress granules regulate double-stranded RNA-dependent protein kinase activation through a complex containing G3BP1 and Caprin1.mBio. 2015 Mar 17;6(2):e02486. doi: 10.1128/mBio.02486-14. mBio. 2015. PMID: 25784705 Free PMC article.
-
The roles of G3BP1 in human diseases (review).Gene. 2022 May 5;821:146294. doi: 10.1016/j.gene.2022.146294. Epub 2022 Feb 14. Gene. 2022. PMID: 35176431 Review.
-
Regulation of ribonucleoprotein condensates by RNase L during viral infection.Wiley Interdiscip Rev RNA. 2023 Jul-Aug;14(4):e1770. doi: 10.1002/wrna.1770. Epub 2022 Dec 7. Wiley Interdiscip Rev RNA. 2023. PMID: 36479619 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

