KLRG1-expressing CD8+ T cells are exhausted and polyfunctional in patients with chronic hepatitis B
- PMID: 38776335
- PMCID: PMC11111010
- DOI: 10.1371/journal.pone.0303945
KLRG1-expressing CD8+ T cells are exhausted and polyfunctional in patients with chronic hepatitis B
Abstract
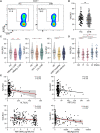
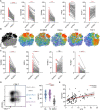
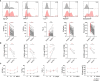
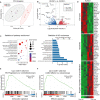
Killer cell lectin-like receptor G1 (KLRG1) has traditionally been regarded as an inhibitory receptor of T cell exhaustion in chronic infection and inflammation. However, its exact role in hepatitis B virus (HBV) infection remains elusive. CD8+ T cells from 190 patients with chronic hepatitis B were analyzed ex vivo for checkpoint and apoptosis markers, transcription factors, cytokines and subtypes in 190 patients with chronic hepatitis B. KLRG1+ and KLRG1- CD8+ T cells were sorted for transcriptome analysis. The impact of the KLRG1-E-cadherin pathway on the suppression of HBV replication mediated by virus-specific T cells was validated in vitro. As expected, HBV-specific CD8+ T cells expressed higher levels of KLRG1 and showed an exhausted molecular phenotype and function. However, despite being enriched for the inhibitory molecules, thymocyte selection-associated high mobility group box protein (TOX), eomesodermin (EOMES), and Helios, CD8+ T cells expressing KLRG1 produced significant levels of tumour necrosis factor (TNF)-α, interferon (IFN)-γ, perforin, and granzyme B, demonstrating not exhausted but active function. Consistent with the in vitro phenotypic assay results, RNA sequencing (RNA-seq) data showed that signature effector T cell and exhausted T cell genes were enriched in KLRG1+ CD8+ T cells. Furthermore, in vitro testing confirmed that KLRG1-E-cadherin binding inhibits the antiviral efficacy of HBV-specific CD8+ T cells. Based on these findings, we concluded that KLRG1+ CD8+ T cells are not only a terminally exhausted subgroup but also exhibit functional diversity, despite inhibitory signs in HBV infection.
Copyright: © 2024 Wang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
KLRG1+ natural killer cells exert a novel antifibrotic function in chronic hepatitis B.J Hepatol. 2019 Aug;71(2):252-264. doi: 10.1016/j.jhep.2019.03.012. Epub 2019 Mar 21. J Hepatol. 2019. PMID: 30905683
-
Cytotoxic KLRG1 expressing lymphocytes invade portal tracts in primary biliary cholangitis.J Autoimmun. 2019 Sep;103:102293. doi: 10.1016/j.jaut.2019.06.004. Epub 2019 Jun 27. J Autoimmun. 2019. PMID: 31255417 Clinical Trial.
-
CXCL13-mediated recruitment of intrahepatic CXCR5+CD8+ T cells favors viral control in chronic HBV infection.J Hepatol. 2020 Mar;72(3):420-430. doi: 10.1016/j.jhep.2019.09.031. Epub 2019 Oct 11. J Hepatol. 2020. PMID: 31610223
-
T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance.Cell Death Dis. 2015 Mar 19;6(3):e1694. doi: 10.1038/cddis.2015.42. Cell Death Dis. 2015. PMID: 25789969 Free PMC article. Review.
-
The role of KLRG1: a novel biomarker and new therapeutic target.Cell Commun Signal. 2024 Jun 19;22(1):337. doi: 10.1186/s12964-024-01714-7. Cell Commun Signal. 2024. PMID: 38898461 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

