This is a preprint.
Elevating levels of the endocannabinoid 2-arachidonoylglycerol blunts opioid reward but not analgesia
- PMID: 38766079
- PMCID: PMC11101127
- DOI: 10.1101/2024.04.02.585967
Elevating levels of the endocannabinoid 2-arachidonoylglycerol blunts opioid reward but not analgesia
Update in
-
Elevating levels of the endocannabinoid 2-arachidonoylglycerol blunts opioid reward but not analgesia.Sci Adv. 2024 Nov 29;10(48):eadq4779. doi: 10.1126/sciadv.adq4779. Epub 2024 Nov 29. Sci Adv. 2024. PMID: 39612328 Free PMC article.
Abstract
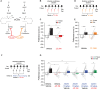
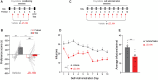
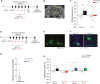
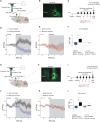
Converging findings have established that the endocannabinoid (eCB) system serves as a possible target for the development of new treatments for pain as a complement to opioid-based treatments. Here we show in male and female mice that enhancing levels of the eCB, 2-arachidonoylglycerol (2-AG), through pharmacological inhibition of its catabolic enzyme, monoacylglycerol lipase (MAGL), either systemically or in the ventral tegmental area (VTA) with JZL184, leads to a substantial attenuation of the rewarding effects of opioids in male and female mice using conditioned place preference and self-administration paradigms, without altering their analgesic properties. These effects are driven by CB1 receptors (CB1Rs) within the VTA as VTA CB1R conditional knockout, counteracts JZL184's effects. Conversely, pharmacologically enhancing the levels of the other eCB, anandamide (AEA), by inhibition of fatty acid amide hydrolase (FAAH) has no effect on opioid reward or analgesia. Using fiber photometry with fluorescent sensors for calcium and dopamine (DA), we find that enhancing 2-AG levels diminishes opioid reward-related nucleus accumbens (NAc) activity and DA neurotransmission. Together these findings reveal that 2-AG counteracts the rewarding properties of opioids and provides a potential adjunctive therapeutic strategy for opioid-related analgesic treatments.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures

Similar articles
-
Elevating levels of the endocannabinoid 2-arachidonoylglycerol blunts opioid reward but not analgesia.Sci Adv. 2024 Nov 29;10(48):eadq4779. doi: 10.1126/sciadv.adq4779. Epub 2024 Nov 29. Sci Adv. 2024. PMID: 39612328 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
References
-
- NIDA. National Institute of Drug Abuse; 2018.
-
- Strang J, et al. Opioid use disorder. Nat Rev Dis Primers. 2020;6(1):3. - PubMed
Publication types
Grants and funding
- R01 DA047851/DA/NIDA NIH HHS/United States
- R01 DA053261/DA/NIDA NIH HHS/United States
- R01 DA042888/DA/NIDA NIH HHS/United States
- R01 MH125006/MH/NIMH NIH HHS/United States
- R01 MH109685/MH/NIMH NIH HHS/United States
- R01 DA049837/DA/NIDA NIH HHS/United States
- R01 DA007242/DA/NIDA NIH HHS/United States
- R21 DA048635/DA/NIDA NIH HHS/United States
- R33 DA051529/DA/NIDA NIH HHS/United States
- T32 DA007242/DA/NIDA NIH HHS/United States
- R01 DA029122/DA/NIDA NIH HHS/United States
- R37 DA007242/DA/NIDA NIH HHS/United States
- R01 DA054368/DA/NIDA NIH HHS/United States
- R01 DA047265/DA/NIDA NIH HHS/United States
- R01 MH123154/MH/NIMH NIH HHS/United States
- R61 DA051529/DA/NIDA NIH HHS/United States
- R01 MH118451/MH/NIMH NIH HHS/United States
- R01 DA042943/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
