B cell heterogeneity in human tuberculosis highlights compartment-specific phenotype and functional roles
- PMID: 38755239
- PMCID: PMC11099031
- DOI: 10.1038/s42003-024-06282-7
B cell heterogeneity in human tuberculosis highlights compartment-specific phenotype and functional roles
Abstract
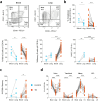
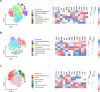
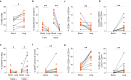
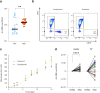
B cells are important in tuberculosis (TB) immunity, but their role in the human lung is understudied. Here, we characterize B cells from lung tissue and matched blood of patients with TB and found they are decreased in the blood and increased in the lungs, consistent with recruitment to infected tissue, where they are located in granuloma associated lymphoid tissue. Flow cytometry and transcriptomics identify multiple B cell populations in the lung, including those associated with tissue resident memory, germinal centers, antibody secretion, proinflammatory atypical B cells, and regulatory B cells, some of which are expanded in TB disease. Additionally, TB lungs contain high levels of Mtb-reactive antibodies, specifically IgM, which promotes Mtb phagocytosis. Overall, these data reveal the presence of functionally diverse B cell subsets in the lungs of patients with TB and suggest several potential localized roles that may represent a target for interventions to promote immunity or mitigate immunopathology.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Understanding the development of tuberculous granulomas: insights into host protection and pathogenesis, a review in humans and animals.Front Immunol. 2024 Dec 9;15:1427559. doi: 10.3389/fimmu.2024.1427559. eCollection 2024. Front Immunol. 2024. PMID: 39717773 Free PMC article. Review.
-
B cells in perivascular and peribronchiolar granuloma-associated lymphoid tissue and B-cell signatures identify asymptomatic Mycobacterium tuberculosis lung infection in Diversity Outbred mice.Infect Immun. 2024 Jul 11;92(7):e0026323. doi: 10.1128/iai.00263-23. Epub 2024 Jun 20. Infect Immun. 2024. PMID: 38899881 Free PMC article.
-
Detailed phenotyping reveals diverse and highly skewed neutrophil subsets in both the blood and airways during active tuberculosis infection.Front Immunol. 2024 Jun 14;15:1422836. doi: 10.3389/fimmu.2024.1422836. eCollection 2024. Front Immunol. 2024. PMID: 38947330 Free PMC article.
-
Community views on active case finding for tuberculosis in low- and middle-income countries: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2024 Mar 21;3(3):CD014756. doi: 10.1002/14651858.CD014756.pub2. Cochrane Database Syst Rev. 2024. PMID: 38511668 Free PMC article. Review.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
Cited by
-
B Cell and Antibody Responses in Bovine Tuberculosis.Antibodies (Basel). 2024 Oct 9;13(4):84. doi: 10.3390/antib13040084. Antibodies (Basel). 2024. PMID: 39449326 Free PMC article. Review.
-
Differential Diagnosis of Tuberculosis and Sarcoidosis by Immunological Features Using Machine Learning.Diagnostics (Basel). 2024 Sep 30;14(19):2188. doi: 10.3390/diagnostics14192188. Diagnostics (Basel). 2024. PMID: 39410592 Free PMC article.
References
-
- Organization, W. H. Global Tuberculosis Report 2022 (2022).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

