Microbiota-derived 3-phenylpropionic acid promotes myotube hypertrophy by Foxo3/NAD+ signaling pathway
- PMID: 38750565
- PMCID: PMC11097579
- DOI: 10.1186/s13578-024-01244-2
Microbiota-derived 3-phenylpropionic acid promotes myotube hypertrophy by Foxo3/NAD+ signaling pathway
Abstract
Background: Gut microbiota and their metabolites play a regulatory role in skeletal muscle growth and development, which be known as gut-muscle axis. 3-phenylpropionic acid (3-PPA), a metabolite produced by colonic microorganisms from phenylalanine in the gut, presents in large quantities in the blood circulation. But few study revealed its function in skeletal muscle development.
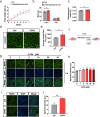
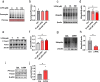
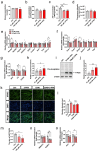
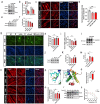
Results: Here, we demonstrated the beneficial effects of 3-PPA on muscle mass increase and myotubes hypertrophy both in vivo and vitro. Further, we discovered the 3-PPA effectively inhibited protein degradation and promoted protein acetylation in C2C12 and chick embryo primary skeletal muscle myotubes. Mechanistically, we supported that 3-PPA reduced NAD+ synthesis and subsequently suppressed tricarboxylic acid cycle and the mRNA expression of SIRT1/3, thus promoting the acetylation of total protein and Foxo3. Moreover, 3-PPA may inhibit Foxo3 activity by directly binding.
Conclusions: This study firstly revealed the effect of 3-PPA on skeletal muscle growth and development, and newly discovered the interaction between 3-PPA and Foxo3/NAD+ which mechanically promote myotubes hypertrophy. These results expand new understanding for the regulation of gut microbiota metabolites on skeletal muscle growth and development.
Keywords: 3-Phenylpropionic acid; Acetylation; Gut microbiota metabolites; Muscle hypertrophy; NAD+.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Phenylpropionic acid produced by gut microbiota alleviates acetaminophen-induced hepatotoxicity.Gut Microbes. 2023 Jan-Dec;15(1):2231590. doi: 10.1080/19490976.2023.2231590. Gut Microbes. 2023. PMID: 37431867 Free PMC article.
-
Foxo3 Knockdown Mediates Decline of Myod1 and Myog Reducing Myoblast Conversion to Myotubes.Cells. 2023 Aug 29;12(17):2167. doi: 10.3390/cells12172167. Cells. 2023. PMID: 37681900 Free PMC article.
-
Mannan Oligosaccharides Promoted Skeletal Muscle Hypertrophy through the Gut Microbiome and Microbial Metabolites in Mice.Foods. 2023 Jan 12;12(2):357. doi: 10.3390/foods12020357. Foods. 2023. PMID: 36673449 Free PMC article.
-
PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review.
-
Gut microbiota-bile acid-skeletal muscle axis.Trends Microbiol. 2023 Mar;31(3):254-269. doi: 10.1016/j.tim.2022.10.003. Epub 2022 Oct 29. Trends Microbiol. 2023. PMID: 36319506 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

