Research progress of cell membrane biomimetic nanoparticles for circulating tumor cells
- PMID: 38746681
- PMCID: PMC11091305
- DOI: 10.3389/fonc.2024.1389775
Research progress of cell membrane biomimetic nanoparticles for circulating tumor cells
Abstract
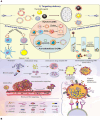
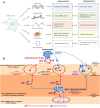
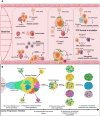
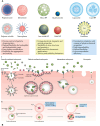
Early detection of cancer is crucial to reducing fatalities and improving patient outcomes. Metastasis is the first stage of aggressive cancers, often occurring before primary lesions can be seen. It occurs when cancerous cells disseminate to distant, non-malignant organs through the bloodstream, known as circulating tumor cells (CTCs). CTCs, or cancer tumor cells, are valuable indicators for predicting treatment response, metastasis progression, and disease progression. However, they are primarily used for research due to challenges like heterogeneity, separation from blood, and lack of clinical validation. Only a few methods have been approved for clinical use. One area of research is the isolation and identification of CTCs, which could significantly impact early cancer detection and prognosis. Current technologies using whole-blood samples use size, immunoaffinity, and density approaches, along with positive and negative enrichment techniques. Surface modification of nanomaterials is important for effective cancer therapies because it improves their ability to target and reduces interactions with healthy tissues. Consequently, researchers have created biomimetic nanoparticles covered with cell membranes using functional, targeted, and biocompatible coating technology. Nanoparticles with membranes can target specific cells, stay in circulation for longer, and avoid immune responses, which makes them much better at capturing CTCs. This study examines the current opportunities and difficulties associated with using cell membrane-coated nanoparticles as a capture technique for CTCs. In addition, we examine potential future developments in light of the current obstacles and investigate areas that require further research to fully understand its growing clinical possibilities.
Keywords: biomimetic nanoparticles; cell membrane; circulating tumor cells; diagnosis; progress.
Copyright © 2024 Zhang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








Similar articles
-
Neutrophil membrane-coated immunomagnetic nanoparticles for efficient isolation and analysis of circulating tumor cells.Biosens Bioelectron. 2022 Oct 1;213:114425. doi: 10.1016/j.bios.2022.114425. Epub 2022 Jun 4. Biosens Bioelectron. 2022. PMID: 35688024
-
Detection of circulating tumor cells: opportunities and challenges.Biomark Res. 2022 Aug 13;10(1):58. doi: 10.1186/s40364-022-00403-2. Biomark Res. 2022. PMID: 35962400 Free PMC article. Review.
-
Surface modification potentials of cell membrane-based materials for targeted therapies: a chemotherapy-focused review.Nanomedicine (Lond). 2023 Aug;18(19):1281-1303. doi: 10.2217/nnm-2023-0164. Epub 2023 Sep 27. Nanomedicine (Lond). 2023. PMID: 37753724 Review.
-
Leukocyte-Repelling Biomimetic Immunomagnetic Nanoplatform for High-Performance Circulating Tumor Cells Isolation.Small. 2019 Apr;15(17):e1900558. doi: 10.1002/smll.201900558. Epub 2019 Apr 1. Small. 2019. PMID: 30932344
-
Circulating Tumor Cells in Gastrointestinal Cancers: Current Status and Future Perspectives.Front Oncol. 2019 Dec 13;9:1427. doi: 10.3389/fonc.2019.01427. eCollection 2019. Front Oncol. 2019. PMID: 31921680 Free PMC article. Review.
References
-
- Cabel L, Berger F, Cottu P, Loirat D, Rampanou A, Brain E, et al. . Clinical utility of circulating tumour cell-based monitoring of late-line chemotherapy for metastatic breast cancer: the randomised CirCe01 trial. Br J Cancer. (2021) 124:1207–13. doi: 10.1038/s41416-020-01227-3 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

