This is a preprint.
Multi-omics delineate growth factor network underlying exercise effects in an Alzheimer's mouse model
- PMID: 38746443
- PMCID: PMC11092636
- DOI: 10.1101/2024.05.02.592289
Multi-omics delineate growth factor network underlying exercise effects in an Alzheimer's mouse model
Abstract
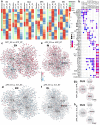
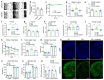
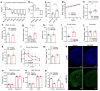
Physical exercise represents a primary defense against age-related cognitive decline and neurodegenerative disorders like Alzheimer's disease (AD). To impartially investigate the underlying mechanisms, we conducted single-nucleus transcriptomic and chromatin accessibility analyses (snRNA-seq and ATAC-seq) on the hippocampus of mice carrying AD-linked NL-G-F mutations in the amyloid precursor protein gene (APPNL-G-F) following prolonged voluntary wheel-running exercise. Our study reveals that exercise mitigates amyloid-induced changes in both transcriptomic expression and chromatin accessibility through cell type-specific transcriptional regulatory networks. These networks converge on the activation of growth factor signaling pathways, particularly the epidermal growth factor receptor (EGFR) and insulin signaling, correlating with an increased proportion of immature dentate granule cells and oligodendrocytes. Notably, the beneficial effects of exercise on neurocognitive functions can be blocked by pharmacological inhibition of EGFR and the downstream phosphoinositide 3-kinases (PI3K). Furthermore, exercise leads to elevated levels of heparin-binding EGF (HB-EGF) in the blood, and intranasal administration of HB-EGF enhances memory function in sedentary APPNL-G-F mice. These findings offer a panoramic delineation of cell type-specific hippocampal transcriptional networks activated by exercise and suggest EGF-related growth factor signaling as a druggable contributor to exercise-induced memory enhancement, thereby suggesting therapeutic avenues for combatting AD-related cognitive decline.
Keywords: Alzheimer’s disease; Physical exercise; cognition; growth factor; hippocampus.
Conflict of interest statement
CONFLICT OF INTEREST The authors declare no financial conflict of interest.
Figures




Similar articles
-
Dramatic impacts on brain pathology, anxiety, and cognitive function in the knock-in APPNL-G-F mouse model of Alzheimer disease following long-term voluntary exercise.Alzheimers Res Ther. 2022 Sep 30;14(1):143. doi: 10.1186/s13195-022-01085-6. Alzheimers Res Ther. 2022. PMID: 36180883 Free PMC article.
-
Long-term running exercise improves cognitive function and promotes microglial glucose metabolism and morphological plasticity in the hippocampus of APP/PS1 mice.J Neuroinflammation. 2022 Feb 5;19(1):34. doi: 10.1186/s12974-022-02401-5. J Neuroinflammation. 2022. PMID: 35123512 Free PMC article.
-
Cognitive and emotional alterations in App knock-in mouse models of Aβ amyloidosis.BMC Neurosci. 2018 Jul 28;19(1):46. doi: 10.1186/s12868-018-0446-8. BMC Neurosci. 2018. PMID: 30055565 Free PMC article.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
Recent Advances in the Modeling of Alzheimer's Disease.Front Neurosci. 2022 Mar 31;16:807473. doi: 10.3389/fnins.2022.807473. eCollection 2022. Front Neurosci. 2022. PMID: 35431779 Free PMC article. Review.
References
-
- McGurran H., Glenn J. M., Madero E. N. & Bott N. T. Prevention and Treatment of Alzheimer’s Disease: Biological Mechanisms of Exercise. J. Alzheimers Dis. JAD 69, 311–338 (2019). - PubMed
-
- Prakash R. S., Voss M. W., Erickson K. I. & Kramer A. F. Physical activity and cognitive vitality. Annu. Rev. Psychol. 66, 769–797 (2015). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
