A two-dose viral-vectored Plasmodium vivax multistage vaccine confers durable protection and transmission-blockade in a pre-clinical study
- PMID: 38745665
- PMCID: PMC11091281
- DOI: 10.3389/fimmu.2024.1372584
A two-dose viral-vectored Plasmodium vivax multistage vaccine confers durable protection and transmission-blockade in a pre-clinical study
Abstract
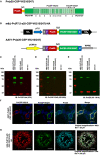
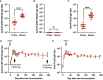
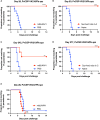
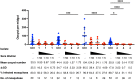
Among Plasmodium spp. responsible for human malaria, Plasmodium vivax ranks as the second most prevalent and has the widest geographical range; however, vaccine development has lagged behind that of Plasmodium falciparum, the deadliest Plasmodium species. Recently, we developed a multistage vaccine for P. falciparum based on a heterologous prime-boost immunization regimen utilizing the attenuated vaccinia virus strain LC16m8Δ (m8Δ)-prime and adeno-associated virus type 1 (AAV1)-boost, and demonstrated 100% protection and more than 95% transmission-blocking (TB) activity in the mouse model. In this study, we report the feasibility and versatility of this vaccine platform as a P. vivax multistage vaccine, which can provide 100% sterile protection against sporozoite challenge and >95% TB efficacy in the mouse model. Our vaccine comprises m8Δ and AAV1 viral vectors, both harboring the gene encoding two P. vivax circumsporozoite (PvCSP) protein alleles (VK210; PvCSP-Sal and VK247; -PNG) and P25 (Pvs25) expressed as a Pvs25-PvCSP fusion protein. For protective efficacy, the heterologous m8Δ-prime/AAV1-boost immunization regimen showed 100% (short-term; Day 28) and 60% (long-term; Day 242) protection against PvCSP VK210 transgenic Plasmodium berghei sporozoites. For TB efficacy, mouse sera immunized with the vaccine formulation showed >75% TB activity and >95% transmission reduction activity by a direct membrane feeding assay using P. vivax isolates in blood from an infected patient from the Brazilian Amazon region. These findings provide proof-of-concept that the m8Δ/AAV1 vaccine platform is sufficiently versatile for P. vivax vaccine development. Future studies are needed to evaluate the safety, immunogenicity, vaccine efficacy, and synergistic effects on protection and transmission blockade in a non-human primate model for Phase I trials.
Keywords: LC16m8Δ; Plasmodium vivax; PvCSP; Pvs25; adeno-associated virus; malaria; vaccine.
Copyright © 2024 Yamamoto, Fabbri, Okuhara, Takagi, Kawabata, Katayama, Iyori, Hasyim, Sakamoto, Mizukami, Shida, Lopes and Yoshida.
Conflict of interest statement
Authors SY, HS, HM, and MI are credited as inventors of patents concerning viral-vectored malaria vaccines 2022-24221. HS is also credited as an inventor on a pending patent related to LC16m8Δ WO 2005/054451 A1. However, neither of these products has been brought to market. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Baculovirus-vectored multistage Plasmodium vivax vaccine induces both protective and transmission-blocking immunities against transgenic rodent malaria parasites.Infect Immun. 2014 Oct;82(10):4348-57. doi: 10.1128/IAI.02040-14. Epub 2014 Aug 4. Infect Immun. 2014. PMID: 25092912 Free PMC article.
-
A universal vaccine candidate against Plasmodium vivax malaria confers protective immunity against the three PvCSP alleles.Sci Rep. 2021 Sep 9;11(1):17928. doi: 10.1038/s41598-021-96986-1. Sci Rep. 2021. PMID: 34504134 Free PMC article.
-
Tailoring a Plasmodium vivax Vaccine To Enhance Efficacy through a Combination of a CSP Virus-Like Particle and TRAP Viral Vectors.Infect Immun. 2018 Aug 22;86(9):e00114-18. doi: 10.1128/IAI.00114-18. Print 2018 Sep. Infect Immun. 2018. PMID: 29986894 Free PMC article.
-
Prime-boost vectored malaria vaccines: progress and prospects.Hum Vaccin. 2010 Jan;6(1):78-83. doi: 10.4161/hv.6.1.10116. Epub 2010 Jan 18. Hum Vaccin. 2010. PMID: 20061802 Review.
-
Transmission-Blocking Vaccines: Harnessing Herd Immunity for Malaria Elimination.Expert Rev Vaccines. 2021 Feb;20(2):185-198. doi: 10.1080/14760584.2021.1878028. Epub 2021 Jan 31. Expert Rev Vaccines. 2021. PMID: 33478283 Free PMC article. Review.
Cited by
-
Development and longevity of naturally acquired antibody and memory B cell responses against Plasmodium vivax infection.PLoS Negl Trop Dis. 2024 Oct 24;18(10):e0012600. doi: 10.1371/journal.pntd.0012600. eCollection 2024 Oct. PLoS Negl Trop Dis. 2024. PMID: 39446698 Free PMC article. Review.
References
-
- Organization WH . World Malaria Report 2022. Geneva: World Health Organization; (2022). Available at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria....
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

