An overview on glycation: molecular mechanisms, impact on proteins, pathogenesis, and inhibition
- PMID: 38737201
- PMCID: PMC11078917
- DOI: 10.1007/s12551-024-01188-4
An overview on glycation: molecular mechanisms, impact on proteins, pathogenesis, and inhibition
Abstract
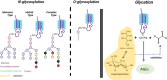
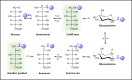
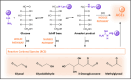
The formation of a heterogeneous set of advanced glycation end products (AGEs) is the final outcome of a non-enzymatic process that occurs in vivo on long-life biomolecules. This process, known as glycation, starts with the reaction between reducing sugars, or their autoxidation products, with the amino groups of proteins, DNA, or lipids, thus gaining relevance under hyperglycemic conditions. Once AGEs are formed, they might affect the biological function of the biomacromolecule and, therefore, induce the development of pathophysiological events. In fact, the accumulation of AGEs has been pointed as a triggering factor of obesity, diabetes-related diseases, coronary artery disease, neurological disorders, or chronic renal failure, among others. Given the deleterious consequences of glycation, evolution has designed endogenous mechanisms to undo glycation or to prevent it. In addition, many exogenous molecules have also emerged as powerful glycation inhibitors. This review aims to provide an overview on what glycation is. It starts by explaining the similarities and differences between glycation and glycosylation. Then, it describes in detail the molecular mechanism underlying glycation reactions, and the bio-molecular targets with higher propensity to be glycated. Next, it discusses the precise effects of glycation on protein structure, function, and aggregation, and how computational chemistry has provided insights on these aspects. Finally, it reports the most prevalent diseases induced by glycation, and the endogenous mechanisms and the current therapeutic interventions against it.
Keywords: Diabetic-related diseases; Glycation; Glycation inhibitors; Protein aggregation; Protein function; Protein structure.
© The Author(s) 2024.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures
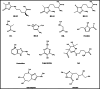
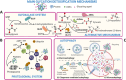
 ) are those that can inhibit glycation through the protection of the amino groups that are prompt to be glycated. The compounds labeled with a blue circle (
) are those that can inhibit glycation through the protection of the amino groups that are prompt to be glycated. The compounds labeled with a blue circle (
 ) are those that can inhibit glycation via scavenging carbonyl compounds. The compounds labeled with a green circle (
) are those that can inhibit glycation via scavenging carbonyl compounds. The compounds labeled with a green circle (
 ) are those that can inhibit glycation via chelating metal cations that promote it. The compounds labeled with a purple circle (
) are those that can inhibit glycation via chelating metal cations that promote it. The compounds labeled with a purple circle (
 ) are those that can inhibit glycation via the neutralization of ROS. The compounds labeled with a yellow circle (
) are those that can inhibit glycation via the neutralization of ROS. The compounds labeled with a yellow circle (
 ) are those that can act as AGE breakers
) are those that can act as AGE breakersSimilar articles
-
A review on prevention of glycation of proteins: Potential therapeutic substances to mitigate the severity of diabetes complications.Int J Biol Macromol. 2022 Jan 15;195:565-588. doi: 10.1016/j.ijbiomac.2021.12.041. Epub 2021 Dec 14. Int J Biol Macromol. 2022. PMID: 34920073 Review.
-
Glycation of Lysozyme by Glycolaldehyde Provides New Mechanistic Insights in Diabetes-Related Protein Aggregation.ACS Chem Biol. 2017 Apr 21;12(4):1152-1162. doi: 10.1021/acschembio.6b01103. Epub 2017 Mar 14. ACS Chem Biol. 2017. PMID: 28257177
-
Glycation Damage: A Possible Hub for Major Pathophysiological Disorders and Aging.Aging Dis. 2018 Oct 1;9(5):880-900. doi: 10.14336/AD.2017.1121. eCollection 2018 Oct. Aging Dis. 2018. PMID: 30271665 Free PMC article. Review.
-
Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives.Biomolecules. 2022 Apr 4;12(4):542. doi: 10.3390/biom12040542. Biomolecules. 2022. PMID: 35454131 Free PMC article. Review.
-
Implication of advanced glycation end products (Ages) and their receptor (Rage) on myocardial contractile and mitochondrial functions.Glycoconj J. 2016 Aug;33(4):607-17. doi: 10.1007/s10719-016-9679-x. Epub 2016 Jun 8. Glycoconj J. 2016. PMID: 27277623 Review.
Cited by
-
Olea europaea L. Leaves as a Source of Anti-Glycation Compounds.Molecules. 2024 Sep 14;29(18):4368. doi: 10.3390/molecules29184368. Molecules. 2024. PMID: 39339362 Free PMC article.
References
-
- Ahmad MI, Ahmad S, Moinuddin M. Preferential recognition of methylglyoxal-modified calf thymus DNA by circulating antibodies in cancer patients. Indian J Biochem Biophys. 2011;48(4):290–296. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

