A Molecular Perspective and Role of NAD+ in Ovarian Aging
- PMID: 38731898
- PMCID: PMC11083308
- DOI: 10.3390/ijms25094680
A Molecular Perspective and Role of NAD+ in Ovarian Aging
Abstract
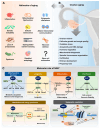
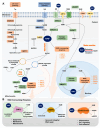
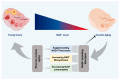
The decline in female fecundity is linked to advancing chronological age. The ovarian reserve diminishes in quantity and quality as women age, impacting reproductive efficiency and the aging process in the rest of the body. NAD+ is an essential coenzyme in cellular energy production, metabolism, cell signaling, and survival. It is involved in aging and is linked to various age-related conditions. Hallmarks associated with aging, diseases, and metabolic dysfunctions can significantly affect fertility by disturbing the delicate relationship between energy metabolism and female reproduction. Enzymes such as sirtuins, PARPs, and CD38 play essential roles in NAD+ biology, which actively consume NAD+ in their enzymatic activities. In recent years, NAD+ has gained much attention for its role in aging and age-related diseases like cancer, Alzheimer's, cardiovascular diseases, and neurodegenerative disorders, highlighting its involvement in various pathophysiological processes. However, its impact on female reproduction is not well understood. This review aims to bridge this knowledge gap by comprehensively exploring the complex interplay between NAD+ biology and female reproductive aging and providing valuable information that could help develop plans to improve women's reproductive health and prevent fertility issues.
Keywords: NAD+ metabolism; aging hallmarks; female fertility; ovarian aging.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
NAD+ and sirtuins in aging and disease.Trends Cell Biol. 2014 Aug;24(8):464-71. doi: 10.1016/j.tcb.2014.04.002. Epub 2014 Apr 29. Trends Cell Biol. 2014. PMID: 24786309 Free PMC article. Review.
-
NAD+, Sirtuins and PARPs: enhancing oocyte developmental competence.J Reprod Dev. 2022 Dec 19;68(6):345-354. doi: 10.1262/jrd.2022-052. Epub 2022 Sep 27. J Reprod Dev. 2022. PMID: 36171094 Free PMC article. Review.
-
Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility.Hum Reprod Update. 2018 May 1;24(3):267-289. doi: 10.1093/humupd/dmy003. Hum Reprod Update. 2018. PMID: 29447380 Review.
-
Hypothalamic NAD+-Sirtuin Axis: Function and Regulation.Biomolecules. 2020 Mar 4;10(3):396. doi: 10.3390/biom10030396. Biomolecules. 2020. PMID: 32143417 Free PMC article. Review.
-
Sirtuin Functions in Female Fertility: Possible Role in Oxidative Stress and Aging.Oxid Med Cell Longev. 2015;2015:659687. doi: 10.1155/2015/659687. Epub 2015 May 5. Oxid Med Cell Longev. 2015. PMID: 26075037 Free PMC article. Review.
Cited by
-
Can we manipulate the ovary's own metabolism to protect it from chemotherapy-induced damage?EMBO Mol Med. 2024 Oct;16(10):2274-2275. doi: 10.1038/s44321-024-00124-z. Epub 2024 Sep 3. EMBO Mol Med. 2024. PMID: 39227717 Free PMC article.
-
Apigenin as a Promising Agent for Enhancing Female Reproductive Function and Treating Associated Disorders.Biomedicines. 2024 Oct 21;12(10):2405. doi: 10.3390/biomedicines12102405. Biomedicines. 2024. PMID: 39457717 Free PMC article. Review.
References
-
- McGee E.A., Hsueh A.J. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000;21:200–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

