Updates in Cancer Cachexia: Clinical Management and Pharmacologic Interventions
- PMID: 38730648
- PMCID: PMC11083841
- DOI: 10.3390/cancers16091696
Updates in Cancer Cachexia: Clinical Management and Pharmacologic Interventions
Abstract
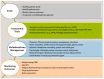
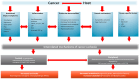
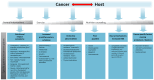
Despite a better understanding of the mechanisms causing cancer cachexia (CC) and development of promising pharmacologic and supportive care interventions, CC persists as an underdiagnosed and undertreated condition. CC contributes to fatigue, poor quality of life, functional impairment, increases treatment related toxicity, and reduces survival. The core elements of CC such as weight loss and poor appetite should be identified early. Currently, addressing contributing conditions (hypothyroidism, hypogonadism, and adrenal insufficiency), managing nutrition impact symptoms leading to decreased oral intake (nausea, constipation, dysgeusia, stomatitis, mucositis, pain, fatigue, depressed mood, or anxiety), and the addition of pharmacologic agents when appropriate (progesterone analog, corticosteroids, and olanzapine) is recommended. In Japan, the clinical practice has changed based on the availability of Anamorelin, a ghrelin receptor agonist that improved lean body mass, weight, and appetite-related quality of life (QoL) compared to a placebo, in phase III trials. Other promising therapeutic agents currently in trials include Espindolol, a non-selective β blocker and a monoclonal antibody to GDF-15. In the future, a single therapeutic agent or perhaps multiple medications targeting the various mechanisms of CC may prove to be an effective strategy. Ideally, these medications should be incorporated into a multimodal interdisciplinary approach that includes exercise and nutrition.
Keywords: cachexia; cancer; cancer cachexia; wasting syndrome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Prokinetics and ghrelin for the management of cancer cachexia syndrome.Ann Palliat Med. 2019 Jan;8(1):80-85. doi: 10.21037/apm.2018.11.01. Epub 2018 Nov 5. Ann Palliat Med. 2019. PMID: 30525771 Review.
-
[Future Prospects of Cancer Cachexia].Gan To Kagaku Ryoho. 2022 Jul;49(7):719-722. Gan To Kagaku Ryoho. 2022. PMID: 35851337 Japanese.
-
Cancer Cachexia: Cause, Diagnosis, and Treatment.Nutr Clin Pract. 2017 Oct;32(5):599-606. doi: 10.1177/0884533617722986. Epub 2017 Aug 21. Nutr Clin Pract. 2017. PMID: 28825869 Review.
-
The emerging role of anamorelin hydrochloride in the management of patients with cancer anorexia-cachexia.Future Oncol. 2017 Aug;13(20):1767-1783. doi: 10.2217/fon-2017-0141. Epub 2017 Jun 16. Future Oncol. 2017. PMID: 28621564 Review.
-
Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials.Lancet Oncol. 2015 Jan;16(1):108-16. doi: 10.1016/S1470-2045(14)71154-4. Epub 2014 Dec 16. Lancet Oncol. 2015. PMID: 25524795
Cited by
-
The Prevalence and Prognosis of Cachexia in Patients with Non-Sarcopenic Dysphagia: A Retrospective Cohort Study.Nutrients. 2024 Sep 1;16(17):2917. doi: 10.3390/nu16172917. Nutrients. 2024. PMID: 39275233 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

