Sodium arsenite and arsenic trioxide differently affect the oxidative stress of lymphoblastoid cells: An intricate crosstalk between mitochondria, autophagy and cell death
- PMID: 38728286
- PMCID: PMC11086853
- DOI: 10.1371/journal.pone.0302701
Sodium arsenite and arsenic trioxide differently affect the oxidative stress of lymphoblastoid cells: An intricate crosstalk between mitochondria, autophagy and cell death
Abstract
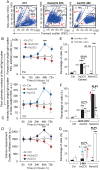
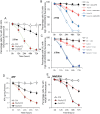
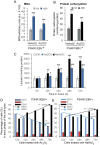
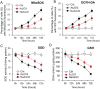
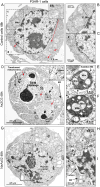
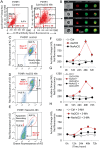
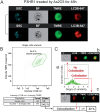
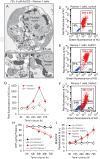
Although the toxicity of arsenic depends on its chemical forms, few studies have taken into account the ambiguous phenomenon that sodium arsenite (NaAsO2) acts as a potent carcinogen while arsenic trioxide (ATO, As2O3) serves as an effective therapeutic agent in lymphoma, suggesting that NaAsO2 and As2O3 may act via paradoxical ways to either promote or inhibit cancer pathogenesis. Here, we compared the cellular response of the two arsenical compounds, NaAsO2 and As2O3, on the Burkitt lymphoma cell model, the Epstein Barr Virus (EBV)-positive P3HR1 cells. Using flow cytometry and biochemistry analyses, we showed that a NaAsO2 treatment induces P3HR1 cell death, combined with drastic drops in ΔΨm, NAD(P)H and ATP levels. In contrast, As2O3-treated cells resist to cell death, with a moderate reduction of ΔΨm, NAD(P)H and ATP. While both compounds block cells in G2/M and affect their protein carbonylation and lipid peroxidation, As2O3 induces a milder increase in superoxide anions and H2O2 than NaAsO2, associated to a milder inhibition of antioxidant defenses. By electron microscopy, RT-qPCR and image cytometry analyses, we showed that As2O3-treated cells display an overall autophagic response, combined with mitophagy and an unfolded protein response, characteristics that were not observed following a NaAsO2 treatment. As previous works showed that As2O3 reactivates EBV in P3HR1 cells, we treated the EBV- Ramos-1 cells and showed that autophagy was not induced in these EBV- cells upon As2O3 treatment suggesting that the boost of autophagy observed in As2O3-treated P3HR1 cells could be due to the presence of EBV in these cells. Overall, our results suggest that As2O3 is an autophagic inducer which action is enhanced when EBV is present in the cells, in contrast to NaAsO2, which induces cell death. That's why As2O3 is combined with other chemicals, as all-trans retinoic acid, to better target cancer cells in therapeutic treatments.
Copyright: © 2024 Rainey et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Sodium arsenite induces apoptosis and Epstein-Barr virus reactivation in lymphoblastoid cells.Biochimie. 2014 Dec;107 Pt B:247-56. doi: 10.1016/j.biochi.2014.09.002. Epub 2014 Sep 21. Biochimie. 2014. PMID: 25241256
-
Critical role of cellular glutathione homeostasis for trivalent inorganic arsenite-induced oxidative damage in human bronchial epithelial cells.Mutat Res Genet Toxicol Environ Mutagen. 2014 Aug;770:35-45. doi: 10.1016/j.mrgentox.2014.04.016. Epub 2014 May 9. Mutat Res Genet Toxicol Environ Mutagen. 2014. PMID: 25344162
-
Sodium arsenite and arsenic trioxide differently affect the oxidative stress, genotoxicity and apoptosis in A549 cells: an implication for the paradoxical mechanism.Environ Toxicol Pharmacol. 2013 Nov;36(3):891-902. doi: 10.1016/j.etap.2013.08.002. Epub 2013 Aug 14. Environ Toxicol Pharmacol. 2013. PMID: 24004876
-
Arsenic trioxide preferentially induces nonapoptotic cell deaths as well as actin cytoskeleton rearrangement in the CHO AA8 cell line.Postepy Hig Med Dosw (Online). 2014 Dec 21;68:1492-500. doi: 10.5604/17322693.1133098. Postepy Hig Med Dosw (Online). 2014. PMID: 25531713 Review.
-
Arsenic trioxide therapy for relapsed acute promyelocytic leukemia: an useful salvage therapy.Leuk Lymphoma. 2000 Jul;38(3-4):283-93. doi: 10.3109/10428190009087019. Leuk Lymphoma. 2000. PMID: 10830735 Review.
References
-
- Miller WH Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62(14):3893–903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

