Retrotransposons in embryogenesis and neurodevelopment
- PMID: 38716891
- PMCID: PMC11346457
- DOI: 10.1042/BST20230757
Retrotransposons in embryogenesis and neurodevelopment
Abstract
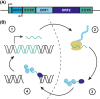
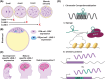
Retrotransposable elements (RTEs) are genetic elements that can replicate and insert new copies into different genomic locations. RTEs have long been identified as 'parasitic genes', as their mobilization can cause mutations, DNA damage, and inflammation. Interestingly, high levels of retrotransposon activation are observed in early embryogenesis and neurodevelopment, suggesting that RTEs may possess functional roles during these stages of development. Recent studies demonstrate that RTEs can function as transcriptional regulatory elements through mechanisms such as chromatin organization and noncoding RNAs. It is clear, however, that RTE expression and activity must be restrained at some level during development, since overactivation of RTEs during neurodevelopment is associated with several developmental disorders. Further investigation is needed to understand the importance of RTE expression and activity during neurodevelopment and the balance between RTE-regulated development and RTE-mediated pathogenesis.
Keywords: LINE-1; neurodevelopment; neurodevelopmental disorders; retrotransposon.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
Similar articles
-
Far Posterior Approach for Rib Fracture Fixation: Surgical Technique and Tips.JBJS Essent Surg Tech. 2024 Dec 6;14(4):e23.00094. doi: 10.2106/JBJS.ST.23.00094. eCollection 2024 Oct-Dec. JBJS Essent Surg Tech. 2024. PMID: 39650795 Free PMC article.
-
Dysregulation of Neurite Outgrowth and Cell Migration in Autism and Other Neurodevelopmental Disorders.Adv Neurobiol. 2020;25:109-153. doi: 10.1007/978-3-030-45493-7_5. Adv Neurobiol. 2020. PMID: 32578146
-
Healthcare workers' informal uses of mobile phones and other mobile devices to support their work: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2024 Aug 27;8(8):CD015705. doi: 10.1002/14651858.CD015705.pub2. Cochrane Database Syst Rev. 2024. PMID: 39189465 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

