Novel 5-HT7 receptor antagonists modulate intestinal immune responses and reduce severity of colitis
- PMID: 38713616
- PMCID: PMC11550998
- DOI: 10.1152/ajpgi.00299.2023
Novel 5-HT7 receptor antagonists modulate intestinal immune responses and reduce severity of colitis
Abstract
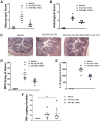
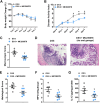
Inflammatory bowel disease (IBD) encompasses several debilitating chronic gastrointestinal (GI) inflammatory disorders, including Crohn's disease and ulcerative colitis. In both conditions, mucosal inflammation is a key clinical presentation associated with altered serotonin (5-hydroxytryptamine or 5-HT) signaling. This altered 5-HT signaling is also found across various animal models of colitis. Of the 14 known receptor subtypes, 5-HT receptor type 7 (5-HT7) is one of the most recently discovered. We previously reported that blocking 5-HT signaling with either a selective 5-HT7 receptor antagonist (SB-269970) or genetic ablation alleviated intestinal inflammation in murine experimental models of colitis. Here, we developed novel antagonists, namely, MC-170073 and MC-230078, which target 5-HT7 receptors with high selectivity. We also investigated the in vivo efficacy of these antagonists in experimental colitis by using dextran sulfate sodium (DSS) and the transfer of CD4+CD45RBhigh T cells to induce intestinal inflammation. Inhibition of 5-HT7 receptor signaling with the antagonists, MC-170073 and MC-230078, ameliorated intestinal inflammation in both acute and chronic colitis models, which was accompanied by lower histopathological damage and diminished levels of proinflammatory cytokines compared with vehicle-treated controls. Together, the data reveal that the pharmacological inhibition of 5-HT7 receptors by these selective antagonists ameliorates the severity of colitis across various experimental models and may, in the future, serve as a potential treatment option for patients with IBD. In addition, these findings support that 5-HT7 is a viable therapeutic target for IBD.NEW & NOTEWORTHY This study demonstrates that the novel highly selective 5-HT7 receptor antagonists, MC-170073 and MC-230078, significantly alleviated the severity of colitis across models of experimental colitis. These findings suggest that inhibition of 5-HT7 receptor signaling by these new antagonists may serve as an alternative mode of treatment to diminish symptomology in those with inflammatory bowel disease.
Keywords: 5-HT; 5-HT7 receptor; 5-HT7 receptor antagonist; inflammatory bowel disease; serotonin.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

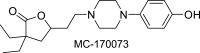

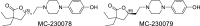

Comment in
-
Targeting serotonin signaling in the gut to limit colitis via 5-HT7 receptor antagonism.Am J Physiol Gastrointest Liver Physiol. 2024 Sep 1;327(3):G454-G455. doi: 10.1152/ajpgi.00181.2024. Epub 2024 Jul 30. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 39076082 No abstract available.
Similar articles
-
Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation.J Immunol. 2013 May 1;190(9):4795-804. doi: 10.4049/jimmunol.1201887. Epub 2013 Apr 3. J Immunol. 2013. PMID: 23554310
-
The tumor necrosis factor family member TNFSF14 (LIGHT) is required for resolution of intestinal inflammation in mice.Gastroenterology. 2014 Jun;146(7):1752-62.e4. doi: 10.1053/j.gastro.2014.02.010. Epub 2014 Feb 19. Gastroenterology. 2014. PMID: 24560868 Free PMC article.
-
Pregnane X receptor agonists enhance intestinal epithelial wound healing and repair of the intestinal barrier following the induction of experimental colitis.Eur J Pharm Sci. 2014 May 13;55:12-9. doi: 10.1016/j.ejps.2014.01.007. Epub 2014 Jan 29. Eur J Pharm Sci. 2014. PMID: 24486481
-
Nociceptin/orphanin FQ inhibition with SB612111 ameliorates dextran sodium sulfate-induced colitis.Eur J Pharmacol. 2012 May 15;683(1-3):285-93. doi: 10.1016/j.ejphar.2012.03.014. Epub 2012 Mar 16. Eur J Pharmacol. 2012. PMID: 22449384 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
References
-
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global. Lancet Gastroenterol Hepatol 5: 17–30, 2020. doi:10.1016/S2468-1253(19)30333-4. - DOI - PMC - PubMed
-
- Windsor JW, Kuenzig ME, Murthy SK, Bitton A, Bernstein CN, Jones JL, Lee K, Targownik LE, Peña-Sánchez J-N, Rohatinsky N, Ghandeharian S, Im JHB, Davis T, Weinstein J, Goddard Q, Benchimol EI, Kaplan GG. The 2023 impact of inflammatory bowel disease in Canada: executive summary. J Can Assoc Gastroenterol 6: S1–S8, 2023. [Erratum in J Can Assoc Gastroenterol 6: 255, 2023]. doi:10.1093/JCAG/GWAD003. - DOI - PMC - PubMed
-
- Crohn’s & Colitis Foundation. Understanding IBD Medications and Side Effects (Online). 2020. https://www.crohnscolitisfoundation.org/sites/default/files/2019-10/unde... [2023 Aug 21].
-
- Mc Gettigan N, Afridi AS, Harkin G, Lardner C, Patchett S, Cheriyan D, Harewood G, Boland K, O'Toole A. The optimal management of anti-drug antibodies to infliximab and identification of anti-drug antibody values for clinical outcomes in patients with inflammatory bowel disease. Int J Colorectal Dis 36: 1231–1241, 2021. doi:10.1007/s00384-021-03855-4. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

