Nuclear and degradative functions of the ESCRT-III pathway: implications for neurodegenerative disease
- PMID: 38700207
- PMCID: PMC11073439
- DOI: 10.1080/19491034.2024.2349085
Nuclear and degradative functions of the ESCRT-III pathway: implications for neurodegenerative disease
Abstract
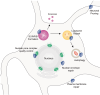
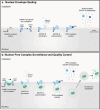
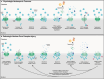
The ESCRT machinery plays a pivotal role in membrane-remodeling events across multiple cellular processes including nuclear envelope repair and reformation, nuclear pore complex surveillance, endolysosomal trafficking, and neuronal pruning. Alterations in ESCRT-III functionality have been associated with neurodegenerative diseases including Frontotemporal Dementia (FTD), Amyotrophic Lateral Sclerosis (ALS), and Alzheimer's Disease (AD). In addition, mutations in specific ESCRT-III proteins have been identified in FTD/ALS. Thus, understanding how disruptions in the fundamental functions of this pathway and its individual protein components in the human central nervous system (CNS) may offer valuable insights into mechanisms underlying neurodegenerative disease pathogenesis and identification of potential therapeutic targets. In this review, we discuss ESCRT components, dynamics, and functions, with a focus on the ESCRT-III pathway. In addition, we explore the implications of altered ESCRT-III function for neurodegeneration with a primary emphasis on nuclear surveillance and endolysosomal trafficking within the CNS.
Keywords: Amyotrophic lateral sclerosis; ESCRT-III pathway; endolysosomal trafficking; frontotemporal dementia; neurodegenerative diseases; nuclear pore complex; nuclear surveillance; protein degradation.
Conflict of interest statement
ANC has submitted patents on methods and drugs to modulate various ESCRT-III proteins in neurodegeneration.
Figures
Similar articles
-
TMEM106B, a frontotemporal lobar dementia (FTLD) modifier, associates with FTD-3-linked CHMP2B, a complex of ESCRT-III.Mol Brain. 2015 Dec 10;8:85. doi: 10.1186/s13041-015-0177-z. Mol Brain. 2015. PMID: 26651479 Free PMC article.
-
Nucleoporins are degraded via upregulation of ESCRT-III/Vps4 complex in Drosophila models of C9-ALS/FTD.Cell Rep. 2022 Sep 20;40(12):111379. doi: 10.1016/j.celrep.2022.111379. Cell Rep. 2022. PMID: 36130523 Free PMC article.
-
Nuclear pore complex and nucleocytoplasmic transport disruption in neurodegeneration.FEBS Lett. 2023 Oct;597(20):2546-2566. doi: 10.1002/1873-3468.14729. Epub 2023 Sep 12. FEBS Lett. 2023. PMID: 37657945 Free PMC article. Review.
-
Lessons learned from CHMP2B, implications for frontotemporal dementia and amyotrophic lateral sclerosis.Neurobiol Dis. 2021 Jan;147:105144. doi: 10.1016/j.nbd.2020.105144. Epub 2020 Nov 2. Neurobiol Dis. 2021. PMID: 33144171 Review.
-
NEMF mutations in mice illustrate how Importin-β specific nuclear transport defects recapitulate neurodegenerative disease hallmarks.PLoS Genet. 2024 Sep 23;20(9):e1011411. doi: 10.1371/journal.pgen.1011411. eCollection 2024 Sep. PLoS Genet. 2024. PMID: 39312574 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
