Inclusion body myositis, viral infections, and TDP-43: a narrative review
- PMID: 38693436
- PMCID: PMC11062973
- DOI: 10.1007/s10238-024-01353-9
Inclusion body myositis, viral infections, and TDP-43: a narrative review
Abstract
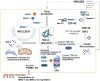
The ubiquitous RNA-processing molecule TDP-43 is involved in neuromuscular diseases such as inclusion body myositis, a late-onset acquired inflammatory myopathy. TDP-43 solubility and function are disrupted in certain viral infections. Certain viruses, high viremia, co-infections, reactivation of latent viruses, and post-acute expansion of cytotoxic T cells may all contribute to inclusion body myositis, mainly in an age-shaped immune landscape. The virally induced senescent, interferon gamma-producing cytotoxic CD8+ T cells with increased inflammatory, and cytotoxic features are involved in the occurrence of inclusion body myositis in most such cases, in a genetically predisposed host. We discuss the putative mechanisms linking inclusion body myositis, TDP-43, and viral infections untangling the links between viruses, interferon, and neuromuscular degeneration could shed a light on the pathogenesis of the inclusion body myositis and other TDP-43-related neuromuscular diseases, with possible therapeutic implications.
Keywords: Inclusion body myositis; Interferon gamma; Long COVID; Myositis triggers; TDP-43.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Undernutrition as a risk factor for tuberculosis disease.Cochrane Database Syst Rev. 2024 Jun 11;6(6):CD015890. doi: 10.1002/14651858.CD015890.pub2. Cochrane Database Syst Rev. 2024. PMID: 38860538 Free PMC article. Review.
-
Pharmacological treatments in panic disorder in adults: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article. Review.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
References
-
- Greenberg SA. Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol. 2019;15(5):257–72. 10.1038/s41584-019-0186-x. - PubMed
-
- McLeish E, Slater N, Sooda A, Wilson A, Coudert JD, Lloyd TE, Needham M. Inclusion body myositis: the interplay between ageing, muscle degeneration and autoimmunity. Best Pract Res Clin Rheumatol. 2022;36(2):101761. 10.1016/j.berh.2022.101761. - PubMed
-
- Lundberg IE, de Visser M, Werth VP. Classification of myositis. Nat Rev Rheumatol. 2018;14(5):269–78. 10.1038/nrrheum.2018.41. - PubMed
-
- Snedden AM, Kellett KAB, Lilleker JB, Hooper NM, Chinoy H. The role of protein aggregation in the pathogenesis of inclusion body myositis. Clin Exp Rheumatol. 2022;40(2):414–24. 10.55563/clinexprheumatol/pp0oso. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials