Exerkine FNDC5/irisin-enriched exosomes promote proliferation and inhibit ferroptosis of osteoblasts through interaction with Caveolin-1
- PMID: 38689463
- PMCID: PMC11320359
- DOI: 10.1111/acel.14181
Exerkine FNDC5/irisin-enriched exosomes promote proliferation and inhibit ferroptosis of osteoblasts through interaction with Caveolin-1
Abstract
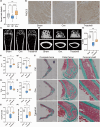
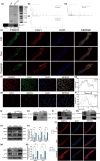
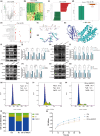
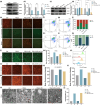
Postmenopausal osteoporosis is a prevalent metabolic bone disorder characterized by a decrease in bone mineral density and deterioration of bone microstructure. Despite the high prevalence of this disease, no effective treatment for osteoporosis has been developed. Exercise has long been considered a potent anabolic factor that promotes bone mass via upregulation of myokines secreted by skeletal muscle, exerting long-term osteoprotective effects and few side effects. Irisin was recently identified as a novel myokine that is significantly upregulated by exercise and could increase bone mass. However, the mechanisms underlying exercise-induced muscle-bone crosstalk remain unclear. Here, we identified that polyunsaturated fatty acids (arachidonic acid and docosahexaenoic acid) are increased in skeletal muscles following a 10-week treadmill exercise programme, which then promotes the expression and release of FNDC5/irisin. In osteoblasts, irisin binds directly to Cav1, which recruits and interacts with AMP-activated protein kinase α (AMPKα) to activate the AMPK pathway. Nrf2 is the downstream target of the AMPK pathway and increases the transcription of HMOX1 and Fpn. HMOX1 is involved in regulating the cell cycle and promotes the proliferation of osteoblasts. Moreover, upregulation of Fpn in osteoblasts enhanced iron removal, thereby suppressing ferroptosis in osteoblasts. Additionally, we confirmed that myotube-derived exosomes are involved in the transportation of irisin and enter osteoblasts through caveolae-mediated endocytosis. In conclusion, our findings highlight the crucial role of irisin, present in myotube-derived exosomes, as a crucial regulator of exercise-induced protective effects on bone, which provides novel insights into the mechanisms underlying exercise-dependent treatment of osteoporosis.
Keywords: Caveolin 1; exercise; exosome; ferroptosis; irisin; osteoporosis.
© 2024 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors are not aware of any affiliations, memberships, funding or financial holdings that might be perceived as affecting the objectivity of this study.
Figures


Similar articles
-
Role of irisin in effects of chronic exercise on muscle and bone in ovariectomized mice.J Bone Miner Metab. 2021 Jul;39(4):547-557. doi: 10.1007/s00774-020-01201-2. Epub 2021 Feb 10. J Bone Miner Metab. 2021. PMID: 33566209
-
Irisin as an agent for protecting against osteoporosis: A review of the current mechanisms and pathways.J Adv Res. 2024 Aug;62:175-186. doi: 10.1016/j.jare.2023.09.001. Epub 2023 Sep 3. J Adv Res. 2024. PMID: 37669714 Free PMC article. Review.
-
Crosstalk Between Muscle and Bone Via the Muscle-Myokine Irisin.Curr Osteoporos Rep. 2016 Aug;14(4):132-7. doi: 10.1007/s11914-016-0313-4. Curr Osteoporos Rep. 2016. PMID: 27299471 Review.
-
Exerkine fibronectin type-III domain-containing protein 5/irisin-enriched extracellular vesicles delay vascular ageing by increasing SIRT6 stability.Eur Heart J. 2022 Nov 14;43(43):4579-4595. doi: 10.1093/eurheartj/ehac431. Eur Heart J. 2022. PMID: 35929617
-
NAD+-boosting therapy alleviates nonalcoholic fatty liver disease via stimulating a novel exerkine Fndc5/irisin.Theranostics. 2021 Feb 25;11(9):4381-4402. doi: 10.7150/thno.53652. eCollection 2021. Theranostics. 2021. PMID: 33754067 Free PMC article.
Cited by
-
The critical roles of caveolin-1 in lung diseases.Front Pharmacol. 2024 Sep 24;15:1417834. doi: 10.3389/fphar.2024.1417834. eCollection 2024. Front Pharmacol. 2024. PMID: 39380904 Free PMC article. Review.
-
Iron homeostasis and ferroptosis in human diseases: mechanisms and therapeutic prospects.Signal Transduct Target Ther. 2024 Oct 14;9(1):271. doi: 10.1038/s41392-024-01969-z. Signal Transduct Target Ther. 2024. PMID: 39396974 Free PMC article. Review.
-
Irisin Protects Musculoskeletal Homeostasis via a Mitochondrial Quality Control Mechanism.Int J Mol Sci. 2024 Sep 20;25(18):10116. doi: 10.3390/ijms251810116. Int J Mol Sci. 2024. PMID: 39337601 Free PMC article. Review.
References
-
- Boström, P. , Wu, J. , Jedrychowski, M. P. , Korde, A. , Ye, L. , Lo, J. C. , Rasbach, K. A. , Boström, E. A. , Choi, J. H. , Long, J. Z. , Kajimura, S. , Zingaretti, M. C. , Vind, B. F. , Tu, H. , Cinti, S. , Højlund, K. , Gygi, S. P. , & Spiegelman, B. M. (2012). A PGC1‐α‐dependent myokine that drives brown‐fat‐like development of white fat and thermogenesis. Nature, 481, 463–468. - PMC - PubMed
-
- Chauhan, S. , Kumar, S. , Jain, A. , Ponpuak, M. , Mudd, M. H. , Kimura, T. , Choi, S. W. , Peters, R. , Mandell, M. , Bruun, J. A. , Johansen, T. , & Deretic, V. (2016). TRIMs and galectins globally cooperate and TRIM16 and Galectin‐3 co‐direct autophagy in endomembrane damage homeostasis. Developmental Cell, 39, 13–27. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

