Modeling the cell biology of monogenetic intestinal epithelial disorders
- PMID: 38683247
- PMCID: PMC11058565
- DOI: 10.1083/jcb.202310118
Modeling the cell biology of monogenetic intestinal epithelial disorders
Abstract
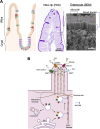
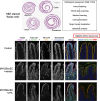
Monogenetic variants are responsible for a range of congenital human diseases. Variants in genes that are important for intestinal epithelial function cause a group of disorders characterized by severe diarrhea and loss of nutrient absorption called congenital diarrheas and enteropathies (CODEs). CODE-causing genes include nutrient transporters, enzymes, structural proteins, and vesicular trafficking proteins in intestinal epithelial cells. Several severe CODE disorders result from the loss-of-function in key regulators of polarized endocytic trafficking such as the motor protein, Myosin VB (MYO5B), as well as STX3, STXBP2, and UNC45A. Investigations of the cell biology and pathophysiology following loss-of-function in these genes have led to an increased understanding of both homeostatic and pathological vesicular trafficking in intestinal epithelial cells. Modeling different CODEs through investigation of changes in patient tissues, coupled with the development of animal models and patient-derived enteroids, has provided critical insights into the enterocyte differentiation and function. Linking basic knowledge of cell biology with the phenotype of specific patient variants is a key step in developing effective treatments for rare monogenetic diseases. This knowledge can also be applied more broadly to our understanding of common epithelial disorders.
© 2024 Kaji et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures


Similar articles
-
Advances in Evaluation of Chronic Diarrhea in Infants.Gastroenterology. 2018 Jun;154(8):2045-2059.e6. doi: 10.1053/j.gastro.2018.03.067. Epub 2018 Apr 12. Gastroenterology. 2018. PMID: 29654747 Free PMC article. Review.
-
An inducible mouse model for microvillus inclusion disease reveals a role for myosin Vb in apical and basolateral trafficking.Proc Natl Acad Sci U S A. 2015 Oct 6;112(40):12408-13. doi: 10.1073/pnas.1516672112. Epub 2015 Sep 21. Proc Natl Acad Sci U S A. 2015. PMID: 26392529 Free PMC article.
-
Altered MYO5B Function Underlies Microvillus Inclusion Disease: Opportunities for Intervention at a Cellular Level.Cell Mol Gastroenterol Hepatol. 2022;14(3):553-565. doi: 10.1016/j.jcmgh.2022.04.015. Epub 2022 Jun 1. Cell Mol Gastroenterol Hepatol. 2022. PMID: 35660026 Free PMC article. Review.
-
Congenital enteropathies involving defects in enterocyte structure or differentiation.Best Pract Res Clin Gastroenterol. 2022 Feb-Mar;56-57:101784. doi: 10.1016/j.bpg.2021.101784. Epub 2022 Jan 4. Best Pract Res Clin Gastroenterol. 2022. PMID: 35331396 Review.
-
A Functional Relationship Between UNC45A and MYO5B Connects Two Rare Diseases With Shared Enteropathy.Cell Mol Gastroenterol Hepatol. 2022;14(2):295-310. doi: 10.1016/j.jcmgh.2022.04.006. Epub 2022 Apr 11. Cell Mol Gastroenterol Hepatol. 2022. PMID: 35421597 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

