Enhanced therapeutic efficacy for glioblastoma immunotherapy with an oncolytic herpes simplex virus armed with anti-PD-1 antibody and IL-12
- PMID: 38681801
- PMCID: PMC11053222
- DOI: 10.1016/j.omton.2024.200799
Enhanced therapeutic efficacy for glioblastoma immunotherapy with an oncolytic herpes simplex virus armed with anti-PD-1 antibody and IL-12
Abstract
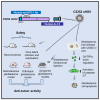
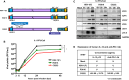
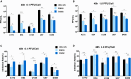
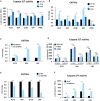
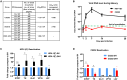
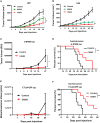
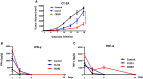
Glioblastoma is the most common and aggressive malignant brain tumor and has limited treatment options. Hence, innovative approaches are urgently needed. Oncolytic virus therapy is emerging as a promising modality for cancer treatment due to its tumor-specific targeting and immune-stimulatory properties. In this study, we developed a new generation of oncolytic herpes simplex virus C5252 by deletion of a 15-kb internal repeat region and both copies of γ34.5 genes. Additionally, C5252 was armed with anti-programmed cell death protein 1 antibody and interleukin-12 to enhance its therapeutic efficacy for glioblastoma immune-virotherapy. In vitro and in vivo experiments demonstrate that C5252 has a remarkable safety profile and potent anti-tumor activity against glioblastoma. Mechanistic studies demonstrated that C5252 specifically induces cell apoptosis by caspase-3/7 activation via downregulating ciliary neurotrophic factor receptor α. Furthermore, the enhanced anti-tumor therapeutic efficacy of C5252 in a subcutaneous glioblastoma model and an orthotopic glioblastoma model was confirmed. Moreover, syngeneic mouse models showed that the murine surrogate of C5252 has superior anti-tumor activity compared to the unarmed backbone virus, with enhanced immune activation. Taken together, our findings support C5252 as a promising therapeutic option for glioblastoma treatment, positioning it as a highly promising candidate for clinical translation.
Keywords: GBM; IL-12; checkpoint inhibitor; glioblastoma; immunotherapy; oHSV; oncolytic herpes simplex virus.
© 2024.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Oncolytic herpes simplex virus expressing IL-2 controls glioblastoma growth and improves survival.J Immunother Cancer. 2024 Apr 9;12(4):e008880. doi: 10.1136/jitc-2024-008880. J Immunother Cancer. 2024. PMID: 38599661 Free PMC article.
-
Implications of immune cells in oncolytic herpes simplex virotherapy for glioma.Brain Tumor Pathol. 2022 Apr;39(2):57-64. doi: 10.1007/s10014-022-00431-8. Epub 2022 Apr 6. Brain Tumor Pathol. 2022. PMID: 35384530 Review.
-
Arming an Oncolytic Herpes Simplex Virus Type 1 with a Single-chain Fragment Variable Antibody against PD-1 for Experimental Glioblastoma Therapy.Clin Cancer Res. 2019 Jan 1;25(1):290-299. doi: 10.1158/1078-0432.CCR-18-2311. Epub 2018 Oct 2. Clin Cancer Res. 2019. PMID: 30279232 Free PMC article.
-
Oncolytic herpes simplex virus immunovirotherapy in combination with immune checkpoint blockade to treat glioblastoma.Immunotherapy. 2018 Jul;10(9):779-786. doi: 10.2217/imt-2018-0009. Immunotherapy. 2018. PMID: 30008259 Free PMC article.
-
The Current State of Oncolytic Herpes Simplex Virus for Glioblastoma Treatment.Oncolytic Virother. 2021 Feb 24;10:1-27. doi: 10.2147/OV.S268426. eCollection 2021. Oncolytic Virother. 2021. PMID: 33659221 Free PMC article. Review.
Cited by
-
A novel oncolytic HSV co-expressing IL-12 and anti-PD-1 for glioblastoma.Mol Ther Oncol. 2024 May 18;32(2):200810. doi: 10.1016/j.omton.2024.200810. eCollection 2024 Jun 20. Mol Ther Oncol. 2024. PMID: 38784951 Free PMC article. No abstract available.
-
Interleukin-12 Delivery Strategies and Advances in Tumor Immunotherapy.Curr Issues Mol Biol. 2024 Oct 16;46(10):11548-11579. doi: 10.3390/cimb46100686. Curr Issues Mol Biol. 2024. PMID: 39451566 Free PMC article. Review.
References
-
- Gatto L., Franceschi E., Tosoni A., Di Nunno V., Bartolini S., Brandes A.A. Glioblastoma treatment slowly moves toward change: novel druggable targets and translational horizons in 2022. Expert Opin. Drug Discov. 2023;18:269–286. - PubMed
-
- Yan G., Wang Y., Chen J., Zheng W., Liu C., Chen S., Wang L., Luo J., Li Z. Advances in drug development for targeted therapies for glioblastoma. Med. Res. Rev. 2020;40:1950–1972. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
