MHC class I-dressing is mediated via phosphatidylserine recognition and is enhanced by polyI:C
- PMID: 38680663
- PMCID: PMC11046299
- DOI: 10.1016/j.isci.2024.109704
MHC class I-dressing is mediated via phosphatidylserine recognition and is enhanced by polyI:C
Abstract
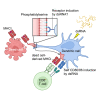
In addition to cross-presentation, cross-dressing plays an important role in the induction of CD8+ T cell immunity. In the process of cross-dressing, conventional dendritic cells (DCs) acquire major histocompatibility complex class I (MHCI) from other cells and subsequently prime CD8+ T cells via the pre-formed antigen-MHCI complexes without antigen processing. However, the mechanisms underlying the cross-dressing pathway, as well as the relative contributions of cross-presentation and cross-dressing to CD8+ T cell priming are not fully understood. Here, we demonstrate that DCs rapidly acquire MHCI-containing membrane fragments from dead cells via the phosphatidylserine recognition-dependent mechanism for cross-dressing. The MHCI dressing is enhanced by a TLR3 ligand polyinosinic-polycytidylic acid (polyI:C). Further, polyI:C promotes not only cross-presentation but also cross-dressing in vivo. Taken together, these results suggest that cross-dressing as well as cross-presentation is involved in inflammatory diseases associated with cell death and type I IFN production.
Keywords: Cell biology; Components of the immune system; Immunology.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells.J Immunol. 2006 Nov 1;177(9):6018-24. doi: 10.4049/jimmunol.177.9.6018. J Immunol. 2006. PMID: 17056526
-
Extracellular vesicle-mediated MHC cross-dressing in immune homeostasis, transplantation, infectious diseases, and cancer.Semin Immunopathol. 2018 Sep;40(5):477-490. doi: 10.1007/s00281-018-0679-8. Epub 2018 Mar 28. Semin Immunopathol. 2018. PMID: 29594331 Free PMC article. Review.
-
MHC-dressing on dendritic cells: Boosting anti-tumor immunity via unconventional tumor antigen presentation.Semin Immunol. 2023 Mar;66:101710. doi: 10.1016/j.smim.2023.101710. Epub 2023 Jan 12. Semin Immunol. 2023. PMID: 36640616 Review.
-
Antigen Presentation by MHC-Dressed Cells.Front Immunol. 2015 Jan 5;5:672. doi: 10.3389/fimmu.2014.00672. eCollection 2014. Front Immunol. 2015. PMID: 25601867 Free PMC article. Review.
-
Shaping of T Cell Functions by Trogocytosis.Cells. 2021 May 10;10(5):1155. doi: 10.3390/cells10051155. Cells. 2021. PMID: 34068819 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

